Question
Question: Consider the following pair of compounds. Which among the following statements are correct? i)Both...
Consider the following pair of compounds. Which among the following statements are correct?
i)Both are enantiomers
ii)Both are in threo form
iii)Both are diastereomers
i)Both are in erythro form
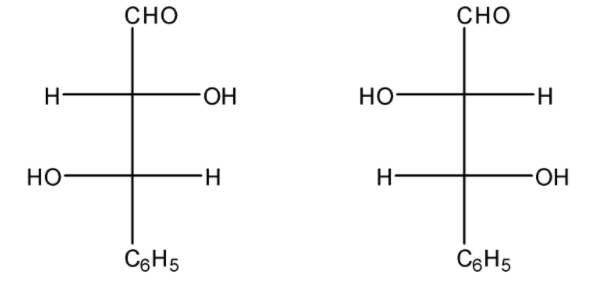
A.i, ii
B.i, ii, iii
C.ii, iii
D.i, iii
Solution
Isomerism is the phenomenon of existence of two or more compounds possessing the same molecular formula but different chemical and physical properties. To answer this question, you must recall the concept of stereoisomerism. Stereoisomerism is defined as the type of isomerism in which compounds possessing the same molecular formula and same structural formula, differ in their properties due to difference in the arrangement in space of their atoms in the molecule.
Complete step by step solution:
Stereoisomerism is defined as the type of isomerism in which compounds possessing the same molecular formula and same structural formula, differ in their properties due to difference in the arrangement in space of their atoms in the molecule. We need to be familiar with the terms given in the question in order to be able to give the answer.
Enantiomers are those stereoisomers which are the non- super imposable mirror images of each other. They exhibit optical activity.
Diastereomers are those stereoisomers which are non- superimposable non- mirror images. They may or may not show optical activity depending on the arrangement of the atoms
Threo and erythro are notations to distinguish stereoisomers. When two identical substituents are present on the same side, the isomer is denoted as erythro and if they are on the opposite sides, then the isomers are denoted as threo.
In the given question, we can clearly see that the given compounds are non- super imposable mirror images of each other. Thus, they are enantiomers. Also the similar groups are present on the opposite sides, so both are in threo.
Statements i and ii are correct.
Thus, the correct option is D.
Note: Isomerism is broadly of two types, that is, structural isomerism or constitutional isomerism, and stereoisomers. Structural isomers are those isomers in which the compounds possessing the same molecular formula differ in their properties due to the difference in the linkage of atoms inside the molecule, i.e., due to a difference in their structures or bonding.
