Question
Question: Consider the following molecules /ions \(\text{ S}{{\text{O}}_{\text{2}}}\text{ }\), \(\text{ C}{{...
Consider the following molecules /ions
SO2 , CO2 , N2O , OF2 , ICl2− , I3− , NO2 , NO2− , NO2+ , BF2− , BeCl2 , N3− , HCN .
Among the above, the number of molecules/ions which are linear is
Solution
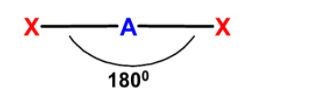
A geometry in which the central atom is bonded to two other atoms such that the bond angle between the bonds is 1800 are known as the linear molecules. In linear geometry, the central atom is sp hybridized. The two-hybrid orbitals involved in the bond formation. The structure is linear. The general structure of the linear molecule is as shown below,
Complete step by step answer:
Let's have a look at the molecules or the ions given in the problem.
A) CO2 : the central carbon atom is bonded to the two oxygen bonds. Carbon has 4 valence electrons in its valence shell .This 4 electrons are involved in the bond formation with oxygen. Thus the CO2 has a linear structure. The bond angle between the bonding pairs is 1800 . The bond is arranged as follows,
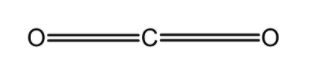
N2O : The central atom nitrogen is asymmetrically bonded to nitrogen and oxygen atoms. The most stable resonating structure, O is more electronegative and has a negative charge and N is less electronegative. Two nitrogen atoms are joined by the triple bond. This is an asymmetric molecule and exhibits a linear structure. The linear structure of N2O or nitrous oxide is as shown below,
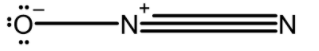
Similarly NO2+ , BeCl2 , N3− and HCN are sp hybridized molecules. They exhibit linear geometry.
ICl2− : The central atom iodine is bonded to two chlorine atoms and acquires three lone pairs of electrons. These lone pairs of electrons are situated in the equatorial plane such that each lone pair is directed towards the corner of a triangle. Thus, iodine is surrounded by 5 electrons pairs. It is sp3d hybridized. Thus the molecular geometry of ICl2− is trigonal bipyramidal. The structure is as shown below,
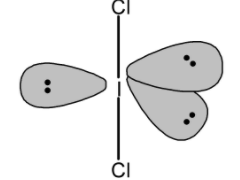
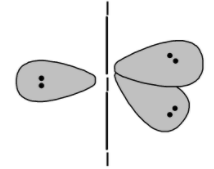
Similarly, the I3− is a sp3d hybridized molecule. The two iodine are bonded to the central iodine atom. The lone three pairs on the iodine atom are placed in the trigonal plane. Thus I3− also has a trigonal bipyramidal geometry. However, the two bonds are in the axial plane make is a linear molecule
B) SO2 : the central sulphur atom is bonded to the two oxygen bonds. Sulphur has 6 valence electrons in its valence shell. Out of these 6 electrons, 4 electrons are involved in the bond formation with oxygen. The two electrons remain as the lone pair on the sulphur atom. The lone pair pushes the bond pairs, thus the SO2 has a trigonal planar. The bond angle between the bonding pairs is 1200 .the structure of SO2 is as follows,
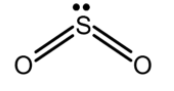
Similarly, the NO2− , NO2 and BF2− are sp2 hybridized molecules but this is non-linear. The lone pair on the atoms makes it trigonal geometry.
CO2 : The central carbon atom is bonded to the two oxygen bonds. Carbon has 4 valence electrons in its valence shell .This 4 electrons are involved in the bond formation with oxygen. Thus the CO2 has a linear structure. The bond angle between the bonding pairs is 1800 . The bond is arranged as follows,
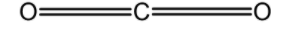
N2O : The central atom nitrogen is asymmetrically bonded to nitrogen and oxygen atoms. The most stable resonating structure, O is more electronegative and has a negative charge and N is less electronegative. Two nitrogen atoms are joined by the triple bond. This is an asymmetric molecule and exhibits a linear structure. The linear structure of N2O or nitrous oxide is as shown below,
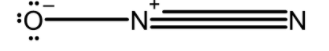
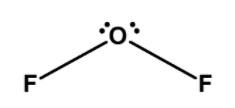
C) OF2 : the central oxygen atom is bonded to the two fluorine bonds. Oxygen has 6 valence electrons in its valence shell. Out of these 6 electrons, 2 electrons are involved in the bond formation with fluorine. The four electrons remain as the lone pair on the oxygen atom. The lone pair pushes the bond pairs, thus OF2 has a trigonal planar. The bond angle between the bonding pairs is 1030 . The structure of OF2 is as follows,
Thus, there are in total eight molecules or ions which have a linear geometry.
Note: Note that, I3− the three iodine atoms and three lone pairs. These lone pairs acquire the equatorial position in such a way that they have zero repulsion between them. In other words, the lone pair on the molecule do not contribute towards the significant geometry. Thus even though the molecule is sp3d hybridized it will be more or a less a linear structure.
