Question
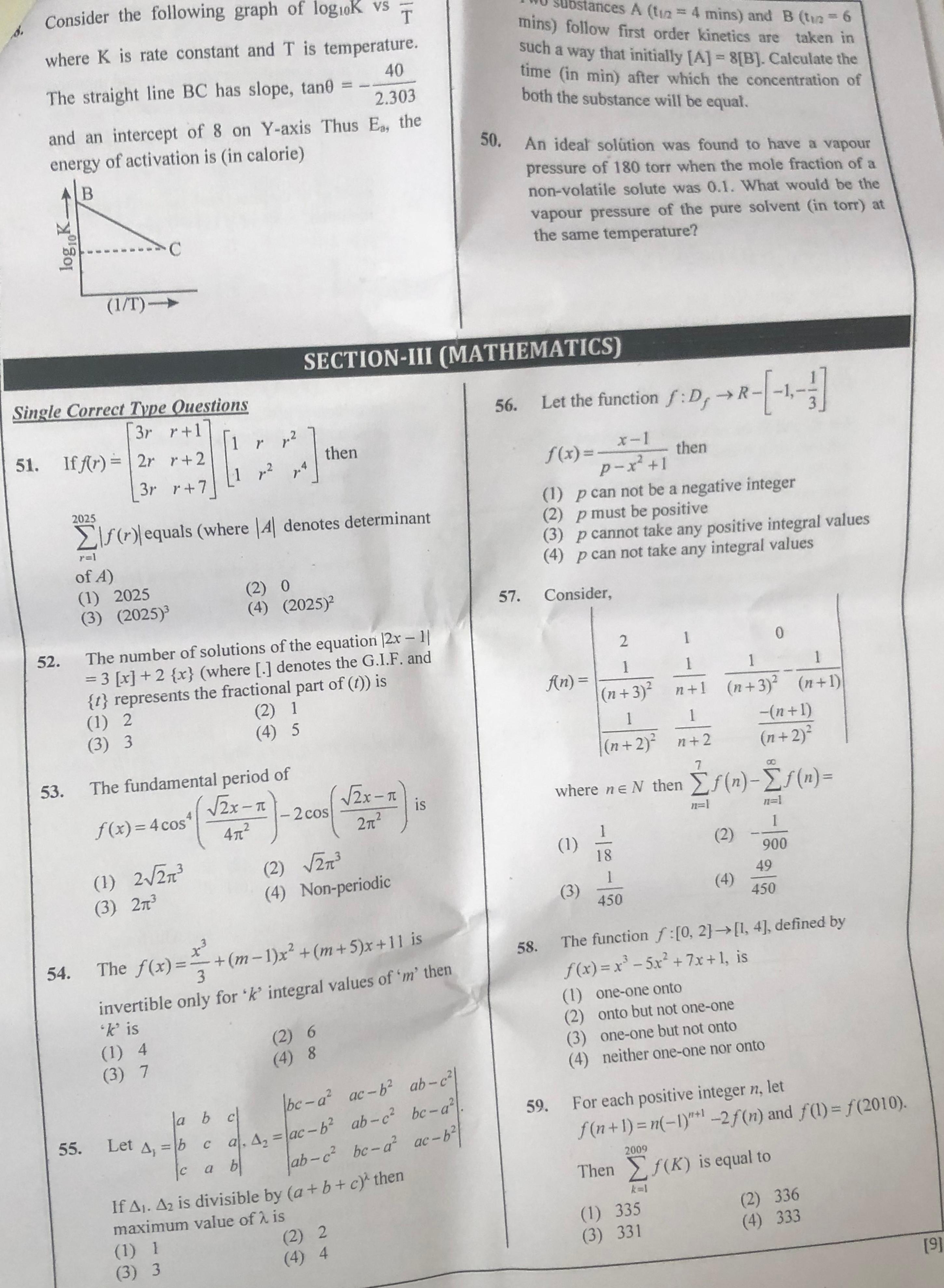
Question: Consider the following graph of log$_{10}$K vs T where K is rate constant and T is temperature. The ...
Consider the following graph of log10K vs T where K is rate constant and T is temperature. The straight line BC has slope, tanθ = -2.30340 and an intercept of 8 on Y-axis Thus Ea, the energy of activation is (in calorie)
80
Solution
The Arrhenius equation is given by k=Ae−Ea/RT. Taking the base-10 logarithm, we get: log10K=log10A−2.303RTEa This equation can be rearranged to represent a straight line when log10K is plotted against 1/T: log10K=(−2.303REa)(T1)+log10A The slope of this line is m=−2.303REa. We are given that the slope tanθ=−2.30340. Equating the slope: −2.303REa=−2.30340 This simplifies to: REa=40 Ea=40R To find Ea in calories, we use the value of the gas constant R≈2cal/(mol⋅K). Ea=40×2cal/mol Ea=80cal/mol The plot of log10K vs 1/T is linear according to the Arrhenius equation log10K=log10A−2.303RTEa. The slope of this line is m=−2.303REa. Given m=−2.30340, we have REa=40. Using R≈2cal/(mol⋅K), Ea=40×2=80cal/mol.
