Question
Question: Consider the following graph and mark the correct statement. <img src="https://cdn.pureessence.tech...
Consider the following graph and mark the correct statement.
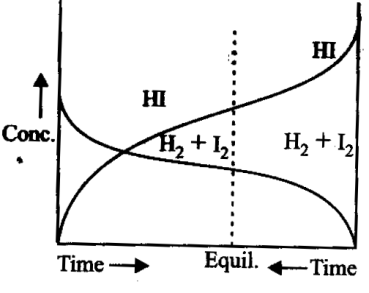
A
Chemical equilibrium in the reaction,
H2+I2 2HI can be attained from either directions.
B
Equilibrium can be obtained when H2and  are mixed in an open vessel.
are mixed in an open vessel.
C
The concentrations of H2and  keep decreasing while concentration of HI keeps increasing with time.
keep decreasing while concentration of HI keeps increasing with time.
D
We can find out equilibrium concentration of H2and I2 from the given graph
Answer
Chemical equilibrium in the reaction,
H2+I2 2HI can be attained from either directions.
Explanation
Solution
: Equlibrium can be attained by either side of the reactons of equilibrium.
