Question
Question: Consider the following experiments for the reaction A → product | Experiment | Time (min) | [A] (M)...
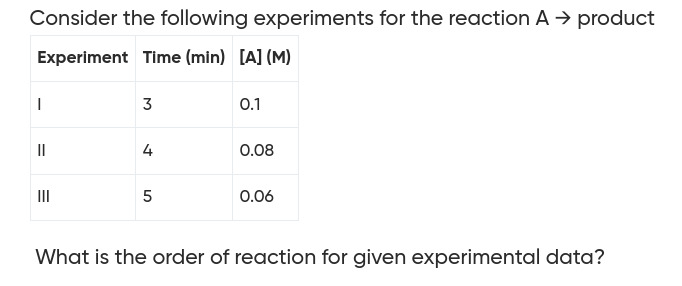
Consider the following experiments for the reaction A → product
| Experiment | Time (min) | [A] (M) |
|---|---|---|
| I | 3 | 0.1 |
| II | 4 | 0.08 |
| III | 5 | 0.06 |
What is the order of reaction for given experimental data?
0
Solution
The problem provides concentration data for reactant A at different times for the reaction A → product. To determine the order of the reaction, we can analyze how the rate of reaction changes with concentration, or by checking if the data fits the integrated rate laws for different orders.
-
Calculate the rate of reaction for each time interval:
The rate of reaction is given by −ΔtΔ[A].-
From Experiment I to Experiment II:
Time interval (Δt) = 4 min−3 min=1 min
Change in [A] (Δ[A]) = 0.08 M−0.1 M=−0.02 M
Rate = −ΔtΔ[A]=−1 min−0.02 M=0.02 M/min -
From Experiment II to Experiment III:
Time interval (Δt) = 5 min−4 min=1 min
Change in [A] (Δ[A]) = 0.06 M−0.08 M=−0.02 M
Rate = −ΔtΔ[A]=−1 min−0.02 M=0.02 M/min
-
-
Determine the order of reaction:
We observe that the rate of reaction is constant (0.02 M/min) across the different time intervals, even though the concentration of A is decreasing.
For a zero-order reaction, the rate of reaction is independent of the concentration of the reactant:
Rate=k[A]0=k
Since the calculated rate is constant, the reaction is a zero-order reaction.
The order of reaction for the given experimental data is 0.
