Question
Question: Consider the following bromides: <img src="https://cdn.pureessence.tech/canvas_66.png?top_left_x=80...
Consider the following bromides:
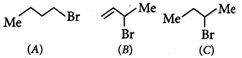
The correct order of SN1reactivity is
A
A>B>C
B
B>C>A
C
B>A>C
D
C>B>A
Answer
B>C>A
Explanation
Solution
SN1reaction rate depends upon the stability of the carbocation as carbocation formation is the rate determining step. Compound (2), forks a 20∘allylic carbocation which is the most stable, the next stable carbocation is formed form (3), it is a 2∘carbocation, (1) foms the least stable 1∘carbocation, the order of reactivity is thus.

