Question
Question: Consider the energy profile, for the reaction \[X + Y \to R + S\] which of the following deductions ...
Consider the energy profile, for the reaction X+Y→R+S which of the following deductions about reaction is not correct?
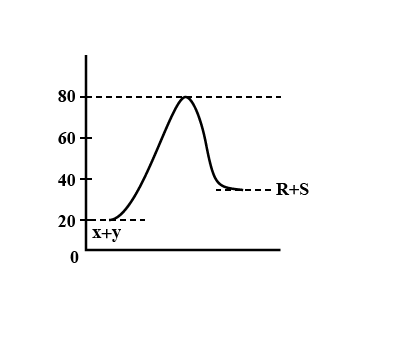
A.The energy of activation for the backward reaction is 80 KJ
B.The forward reaction is Endothermic
C. ΔH for the forward reaction is 20 KJ
D.The energy of activation for forward reaction is 60 KJ
Solution
In the case of an endothermic reaction, the activation energy of the reverse reaction will always be smaller than the activation energy of the reverse reaction and it will be opposite for the exothermic reaction.
Complete step by step answer:
For the reaction X+Y→R+S
The activation energy for the forward reaction, Efa and the activation energy for the backward reaction, Era are related to the enthalpy (ΔH) of the reaction by the equation,
ΔH=Efa−Era
Energy of activation for forward reaction, Efa=80−20=60KJ
Energy of activation for the backward reaction, Era=80−40=40KJ
ΔH=Efa−Era
So ΔH=60−40=20KJ
In the case of an endothermic reaction, the activation energy of the reverse reaction will always be smaller than the activation energy of the reverse reaction.
i.e. for the endothermic reactions, ΔH>0, so that Era<Efa
In the case of an exothermic reaction, the activation energy of the forward reaction will always be smaller than the activation energy of the reverse reaction.
i.e. for the exothermic reactions, ΔH<0, so that Era>Efa
Hence, all the options except the first one is correct.
Therefore, the correct answer is option (A).
Note: In the case of the reverse reaction, we will have to supply energy to the more reactant in order to get it to reform the less stable, i.e. higher in energy product.
The difference between the activation energy of the forward reaction and the activation energy of the reverse reaction will be ΔH. So, for exothermic reactions, we'll always have a bigger Era for the reverse reaction than we had for the forward reaction
