Question
Question: Consider the electrochemical cell M(s)|MI2(s)|MI2(aq) | M(s) where 'M' is a metal. At 298 K, the sta...
Consider the electrochemical cell M(s)|MI2(s)|MI2(aq) | M(s) where 'M' is a metal. At 298 K, the standard reduction potentials are EM2+(aq)/M(s)o=−0.12 V,EMI2(s)/M(s)o=−0.36 V and the temperature coefficient is (∂T∂Ecello)P=1.5×10−4 V K−1. At this temperature the standard enthalpy change for the overall cell reaction, ΔrHP, is __________ kJmol−1. (Faraday constant F=96500Cmol−1)
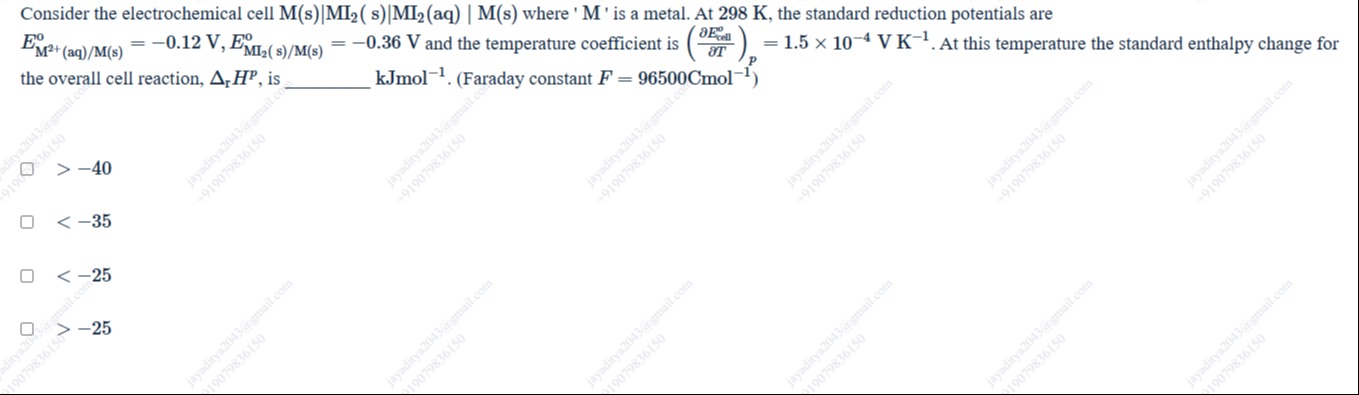
-40
< -35
< -25
-25
< -35
Solution
Here's how to solve this electrochemistry problem:
-
Calculate the standard cell potential (Ecello):
Ecello=Ecathodeo−Eanodeo
Ecello=−0.12 V−(−0.36 V)=0.24 V -
Calculate the standard entropy change (ΔrSo):
ΔrSo=nF(∂T∂Ecello)P
Where:- n=2 (number of moles of electrons transferred)
- F=96500 C mol−1 (Faraday constant)
- (∂T∂Ecello)P=1.5×10−4 V K−1 (temperature coefficient)
ΔrSo=2×96500 C mol−1×1.5×10−4 V K−1=28.95 J K−1mol−1
-
Calculate the standard Gibbs free energy change (ΔrGo):
ΔrGo=−nFEcello
ΔrGo=−2×96500 C mol−1×0.24 V=−46320 J mol−1 -
Calculate the standard enthalpy change (ΔrHo):
ΔrHo=ΔrGo+TΔrSo
ΔrHo=−46320 J mol−1+(298 K×28.95 J K−1mol−1)
ΔrHo=−46320 J mol−1+8627.1 J mol−1=−37692.9 J mol−1
ΔrHo≈−37.7 kJ mol−1
Since −37.7 kJ mol−1 is less than -35 kJ/mol, the correct answer is:
< -35
