Question
Question: Consider the depicted hydrogen (H) in the hydrocarbons given below. The most acidic hydrogen (H) is...
Consider the depicted hydrogen (H) in the hydrocarbons given below. The most acidic hydrogen (H) is
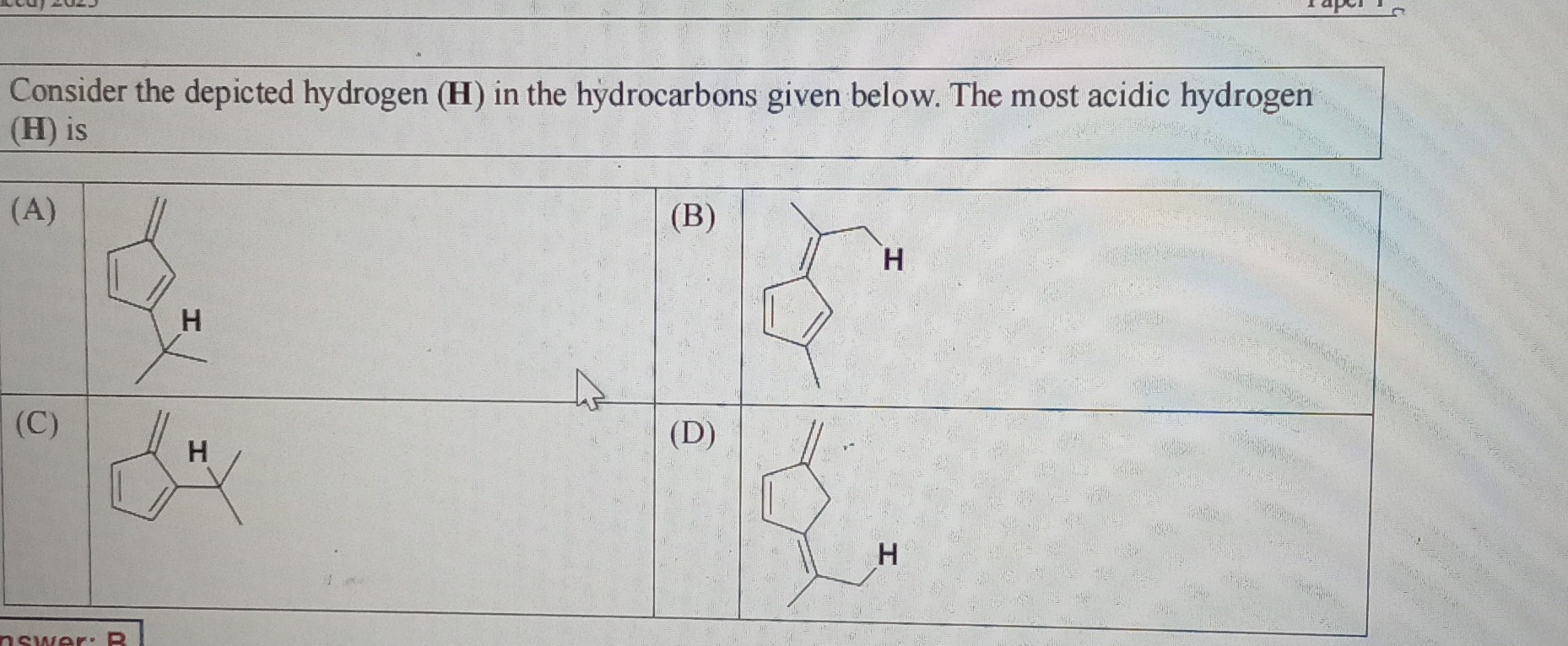
Structure A
Structure B
Structure C
Structure D
Structure A
Solution
To determine the most acidic hydrogen, we need to consider the stability of the conjugate base formed by the removal of the depicted hydrogen. The more stable the conjugate base, the more acidic the hydrogen.
(A) The depicted hydrogen is on the sp3 hybridized carbon of the cyclopentadiene ring. Upon removal of this hydrogen, the cyclopentadienide anion is formed. This anion is aromatic, providing significant stabilization to the conjugate base, making the hydrogens very acidic (pKa ≈ 16).
(B) The depicted hydrogen is on the CH2 group of the side chain attached to the cyclopentadiene ring. Upon removal of this hydrogen, a carbanion is formed, stabilized by resonance. However, this carbanion is not directly conjugated with the π system of the cyclopentadiene ring.
(C) The depicted hydrogen is on a methyl group of the tert-butyl substituent. These hydrogens are not stabilized by resonance or aromaticity.
(D) The depicted hydrogen is on the CH2 group of the side chain attached to a cyclopentene ring. Upon removal of this hydrogen, a carbanion is formed, stabilized by resonance. However, there is no aromatic stabilization from the cyclopentene ring.
Aromatic stabilization is much greater than resonance stabilization. Therefore, the hydrogen in (A), which leads to the aromatic cyclopentadienide anion, is the most acidic among the given options.
