Question
Question: Consider the bulbs shown in the arrangement below. If the pressure of the system when all the stop c...
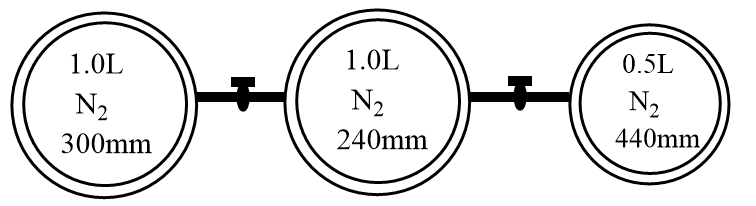
Consider the bulbs shown in the arrangement below. If the pressure of the system when all the stop cocks are opened is x (in atm) then find 100x. (760mm=1atm)
Solution
Boyle’s law which is an experimental gas law which states that the pressure of a gas is inversely proportional to its volume when the number of moles of gas and temperature of the system is considered as constant. In simple words, it describes how pressure of the gas tends to decrease in increasing the volume of the container for an isothermal process.
Complete answer:
For comparing the same gases under two different sets of conditions, the Boyle’s law is expressed as follows:
P1V1=P2V2
Where, P1 and V1 are the pressure and volume of gas at initial conditions while P2 and V2 are the pressure and volume of gas in final conditions.
When two or more moles of gases are mixed, then the Boyle’s will be represented as follows:
PV=P1V1+P2V2
Where P is the pressure of the gas after mixing and V is the final volume of the container which is equal to the sum of the initial volumes i.e., V=V1+V2.
For the given case, the nitrogen gas is present under a different set of conditions in three bulbs. When the stop cork is opened, the gases will start mixing. Assuming the system to be isothermal and ideal mixing of gases, the final volume and pressure can be represented by the following expression:
PV=P1V1+P2V2+P3V3
⇒P(V1+V2+V3)=P1V1+P2V2+P3V3
Substituting given values:
⇒x(1+1+0.5)=300×1+240×1+440×0.5
⇒2.5x=760
⇒x=304mm
Now, it is given in the question that 760mm=1atm. So, converting pressure in unit of atm, the value of x will be as follows:
⇒x=760304
⇒x=0.4atm
Therefore, the value of 100x will be as follows:
100x=100×0.4
⇒100x=40atm
Hence, as per given conditions the value of 100x is 40atm.
Note:
It is important to note that we can alternatively calculate the final pressure of the system by calculating partial pressure of pressure of each set of nitrogen at different condition using ideal gas equation and then according to Dalton’s law of partial pressure, the total pressure of the gas will be equal to the summation of calculated partial pressures.
