Question
Question: Consider the bulbs shown below. If the pressure of the system when all the stop cocks are opened i...
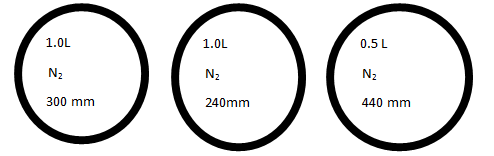
Consider the bulbs shown below.
If the pressure of the system when all the stop cocks are opened is x(in atm) then find100x?
(760mm = 1atm)
Solution
In atmosphere approximately 78% of nitrogen, 21% of oxygen and remaining 1% of other gases in the world. The symbol of nitrogen is N. The natural form of nitrogen gas is diatomic. The symbol of the diatomic nature of nitrogen is N2. The chemical properties of the gas depend on the mixture of gases.
Formula used:
Boyle’s law,
P∝V1 at constant temperature T.
PV = constant
Here, the temperature of the gas is T.
The pressure of the gas P.
The volume of the gas V.
Complete answer:
Boyle’s law states that pressure of the gas P is inversely proportional to volume of the gas V at constant temperature T.
P∝V1 at constant temperature T.
PV = constant
The product of the pressure of the gas and volume of the gas V is always constant at constant temperature T.
P1 = 300mm
P2 = 240mm
P3 = 440mm
V1 = 1.0L
V2 = 1.0L
V3 = 0.5L
The total volume of the container is equal to the sum of the all volume.
V4 = V1 + V2 + V3
Now we can substitute the known values we get,
V4 = 1.0 + 1.0 + 0.5
On simplification we get,
V4 = 2.5L
The total volume of the container is V4and the total pressure of the container isP4.
PV = constant
P4V4 = P1V1 + P2V2 + P3V3
Now we can substitute the known values we get,
P4V4 = 300×1.0 + 240×1.0 + 440×0.5
x×2.5 = 300 + 240 + 220
⇒x = 2.5760
⇒x = 304mm
1atm = 760mm
xatm = 304mm
⇒x = 760304=0.4atm
According to the above calculation,
x = 0.4atm
Then find 100x is
x = 0.4atm
100x = 100×0.4atm
100x = 40atm
Note:
As we know that in chemistry, the periodic table plays a vital role. In the periodic table there are totally 118 elements. In the periodic table there are totally 18 columns and 7 rows. The columns are called groups. Hence, 18 groups in the periodic table. The rows are called periods. Hence, totally 7 period in the table. The atomic number of the element is nothing but the number of electrons or number of protons. The mass number of the atom is nothing but the sum of the number of protons and number of neutrons. The atomic number of nitrogen is 7. The mass number of nitrogen is 14.
