Question
Question: Consider the bcc unit cells of the solids 1 and 2 with the position of atoms as shown below. The rad...
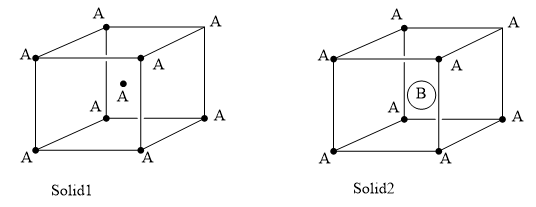
Consider the bcc unit cells of the solids 1 and 2 with the position of atoms as shown below. The radius of atom B is twice that of atom A. The unit cell edge length is 50 more in solid 2 than in 1. What is the approximate packing efficiency in solid 2?
(A)- 45
(B)- 65
(C)- 90
(D)- 75
Solution
The packing efficiency accounts for the fraction of the unit cell occupied by the atoms, by taking the percent of the volume of the total atoms to the volume of the cube.
Complete step by step answer:
It is given that both the solids are body-centred cubic unit cells, that is, there are eight atoms at the corner of the cell and one whole atom in the centre of the unit cell. Each corner atom in the cell is shared among eight other adjacent unit cells. So, the contribution of a single corner atom is (1/8)th in the unit cell. Therefore, having a total eight corner atoms, the total contribution is 8×81= 1 atom. In solid 2, there are eight corner A atoms, and one whole B atom at the centre of the unit cell. The total contribution from the eight corner A atoms is 1 atom and the contribution from the B atom is one. So, two total atoms are present in the unit cell. Then, the packing efficiency, that is the fraction of the unit cell occupied by the atoms is =total volume of the unit cellvolume of the 2 atoms×100 ----------- (a)
-Let the radius of atom A be rA=r, then given that radius of atom B is twice that of A, so rB=2r. ------------ (b)
-Then, the volume of atom A is =34πrA3=34πr3
And volume of atom B is = =34πrB3=34π(2r)3 --------- (c)
-Also, in the bcc unit cell, the relation of the length of body diagonal to the side of the cube is given by: 4r=3a , where r is the radius and a is the length of side of the cube.
-Given, the length of the side of solid 2, a2 is 50 more than a1 of solid 1. So, we have, a2=a1+10050a1=100150a1=23a1
From solid 1, we have the relation 4rA=3a1 or a1=34rA -------- (d)
Substituting the value of a1 in a2, we have a2=23×34rA=23r
-Then volume of the unit cell is a3=(a2)3=(23r)3 ---------- (e)
-Substituting the value of (c) and (e) in equation (a), we get,
Packing efficiency =total volume of solid 2volume of atomA+volumeofatomB×100
=(23r)334πr3+34π(2r)3×100
=8×33r334πr3(1+8)×100=23π×100=90.6
Therefore, the approximate packing efficiency in solid 2 is option (C)- 90
Note: The atoms are taken to be spheres to account for its contribution in the unit cell, so the volume of the sphere is considered. There are a total two atoms in a body-centred cubic unit cell.
