Question
Question: Consider the addition of HBr to 3,3-dimethyl-1-butene shown below. What is the best mechanism explan...
Consider the addition of HBr to 3,3-dimethyl-1-butene shown below. What is the best mechanism explanation for the formation of the observed product?
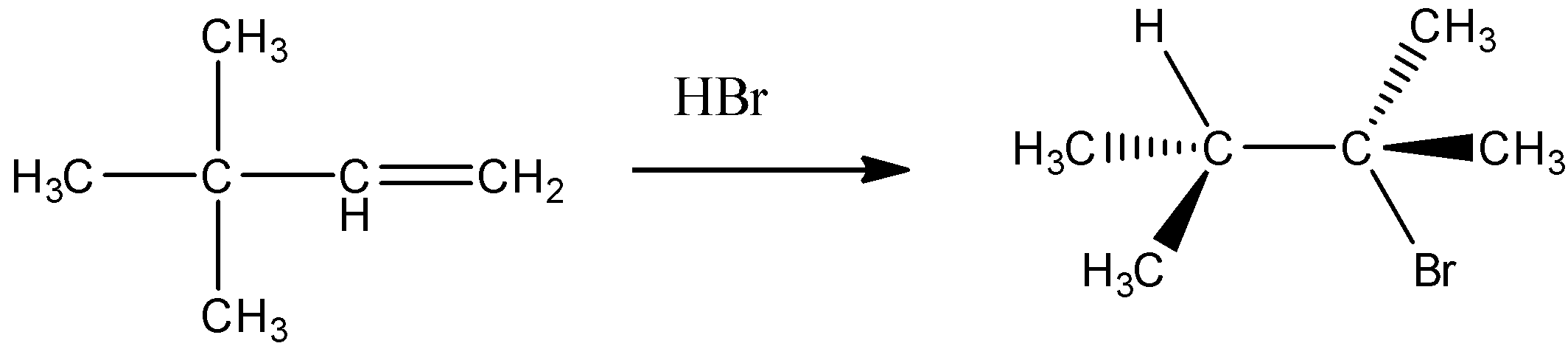
(A) Protonation of the alkene followed by the hydride shift and addition of bromide to the carbocation
(B) Double bond shift in the alkene following by the protonation and addition of bromide to the carbocation
(C) Addition to bromide to the alkene followed by a double bond shift and protonation
(D) Protonation of the alkene followed by a methyl shift and addition of bromide to the carbocation
Solution
Alkene can attack the acid to form carbocation intermediates. The cations formed in the reaction can undergo rearrangement to form more stable carbocation intermediate.
Complete answer:
Let’s see all the steps in the reaction mechanism and understand the reaction first to give the answer.
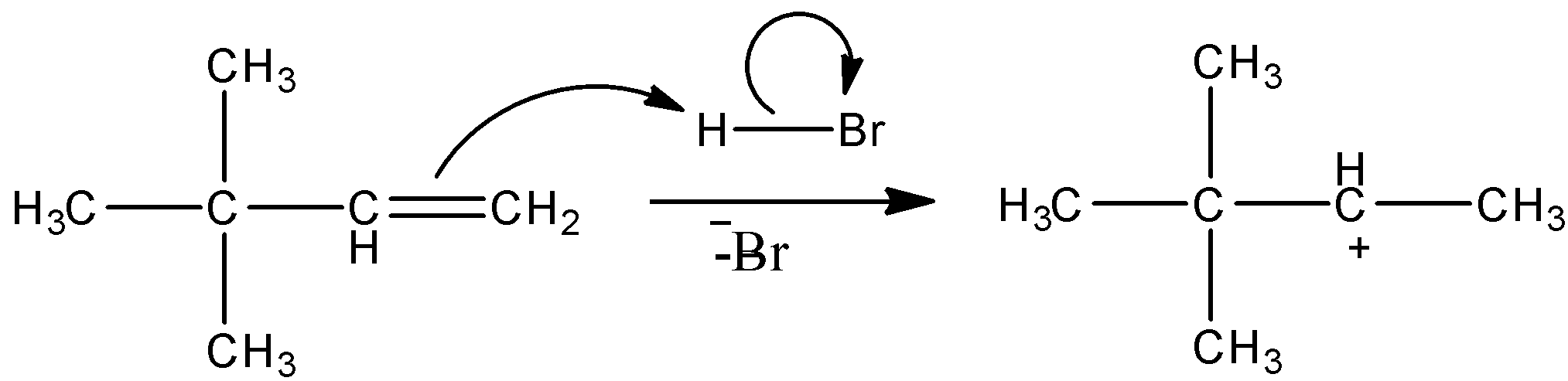
This is the first step of the reaction. All the alkenes will react in this way. They will undergo protonation and will give a carbocation intermediate. Here, the proton gets attached to the terminal carbon because it is primary carbon and according to Markovikov’s rule, proton always gets attached to a carbon that has a higher number of hydrogens. So, we get this carbocation intermediate.
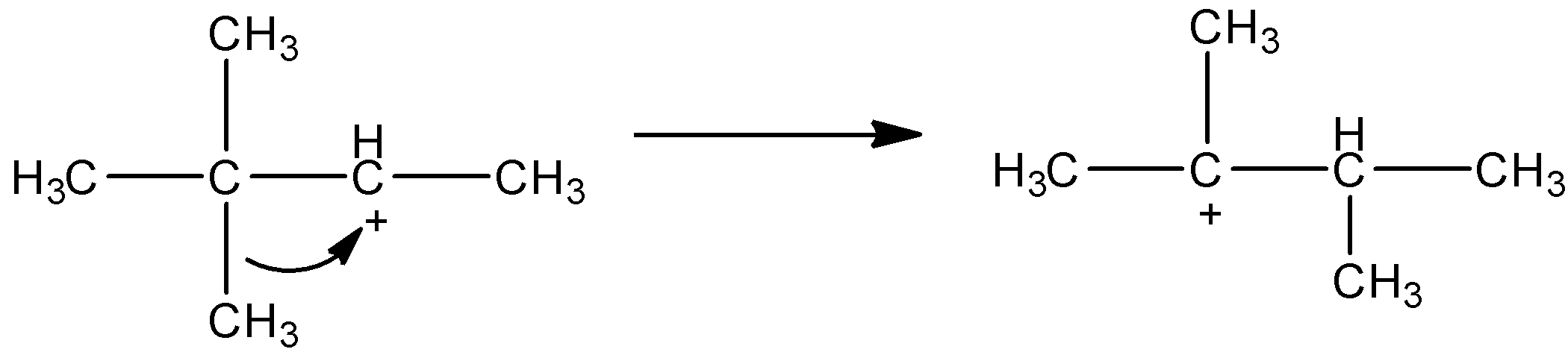
- Now, in this intermediate, you can see that the secondary carbocation is formed. We know that tertiary carbocation is more stable than secondary carbocation. So, if there is possibility of formation of tertiary carbocation by shifting methyl group or hydrogen atoms, then first tertiary carbocation will be formed. Here also we can see in the below mechanism that if the methyl group from the carbon which is α to the carbocation can shift to the cationic carbon and so we will get new cationic carbon which will be tertiary.
- So, as secondary carbocation is less stable, then tertiary carbocation, the formation of tertiary carbocation will take place first and then the bromide will attack the carbocation to give the desired product.
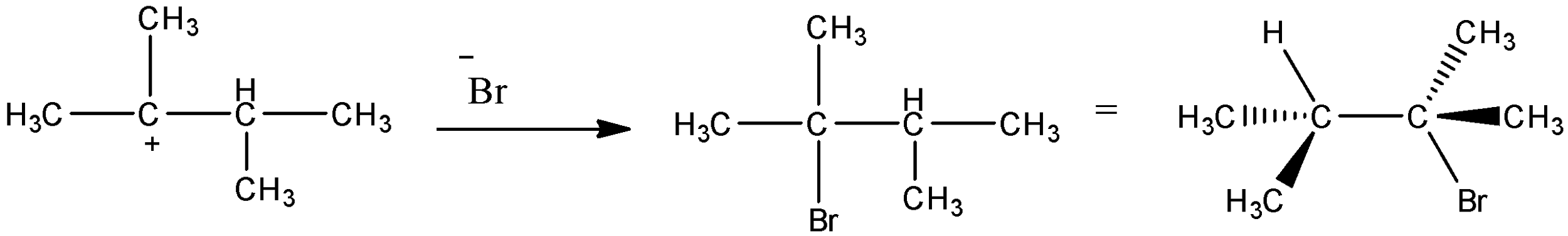
Hence we can say that the correct order of mechanism in this reaction is first protonation of alkene followed by methyl shift and then bromide attacks the carbocation.
So, correct answer is ) Protonation of the alkene followed by a methyl shift and addition of bromide to the carbocation.
Note: Always remember that addition of HBr to any alkene in absence of peroxide always follows Markovnikov’s rule. Remember that shifts of groups only occur when a more stable intermediate is formed as a resulting product.
