Question
Question: Consider following statement: Two chemical structures are shown side-by-side. The first structure ...
Consider following statement:
Two chemical structures are shown side-by-side. The first structure consists of a six-membered ring with alternating single and double bonds, indicating a conjugated system. An -OH (hydroxyl) group is attached to the top carbon of the ring. A positive charge is indicated within the ring. At the bottom of the ring, an H (hydrogen) atom and an -NO2 (nitro) group are attached to the same carbon atom. The second structure consists of a six-membered ring with alternating single and double bonds, indicating a conjugated system. A -CH3 (methyl) group is attached to the top carbon of the ring. A positive charge is indicated within the ring. At the bottom of the ring, an H (hydrogen) atom and an -NO2 (nitro) group are attached to the same carbon atom. (I) is more stable than Two chemical structures are shown side-by-side. The first structure consists of a six-membered ring with alternating single and double bonds, indicating a conjugated system. An -NO2 (nitro) group is attached to the top carbon of the ring. A positive charge is indicated within the ring. At the bottom of the ring, an -OH (hydroxyl) group and a -Cl (chlorine) atom are attached to the same carbon atom. The second structure consists of a six-membered ring with alternating single and double bonds, indicating a conjugated system. An -NO2 (nitro) group is attached to the top carbon of the ring. A negative charge is indicated within the ring. At the bottom of the ring, an -OH (hydroxyl) group and a -Cl (chlorine) atom are attached to the same carbon atom. (II) is more stable than
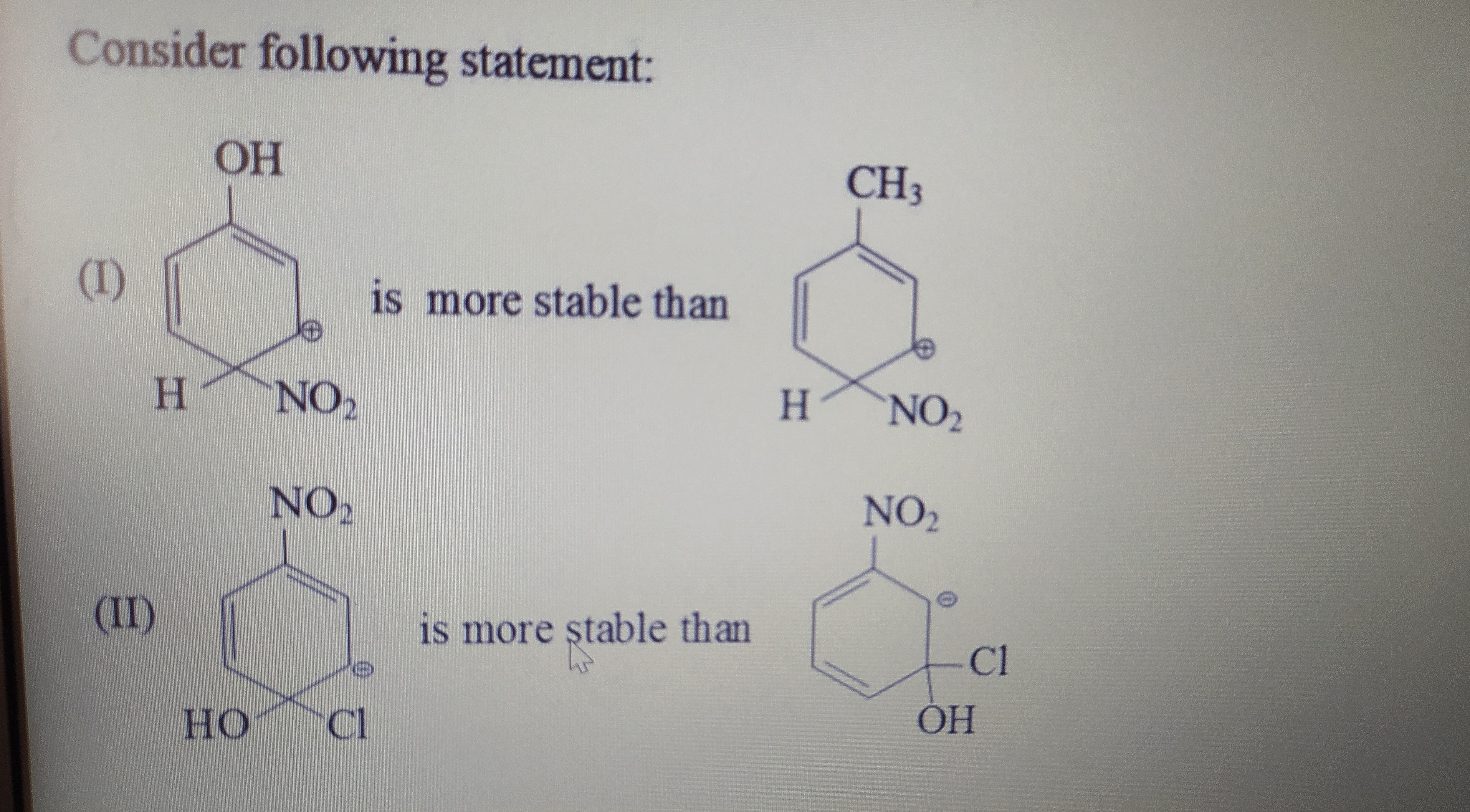
- (I) The arenium ion with –OH is more stable than that with –CH₃.
- (II) The species with the negative charge (in the presence of –NO₂) is more stable than the one with the positive charge.
Solution
-
Comparison (I):
- In the left structure the substituent is –OH, whereas in the right it is –CH₃.
- The –OH group can donate electrons by resonance into the aromatic ring, thereby delocalizing and stabilizing the positive charge.
- A –CH₃ group, although slightly electron‐donating inductively (via hyperconjugation), does not stabilize the positive charge as effectively.
- Thus, the –OH–substituted arenium ion is more stable than the –CH₃–substituted one.
-
Comparison (II):
- Here the top substituent in both structures is –NO₂.
- In one structure the ring carries a positive charge, while in the other it carries a negative charge.
- The nitro group (–NO₂) is strongly electron‐withdrawing by both induction and resonance. It destabilizes a positive charge but helps to stabilize a negative charge by delocalizing electron density.
- Therefore, the structure with the negative charge is more stable than the one with the positive charge.
Minimal Explanation:
- (I) –OH donates electrons by resonance to stabilize a positive charge better than –CH₃.
- (II) A –NO₂ group withdraws electrons, so a negative charge in the ring is stabilized compared to a positive charge.
