Question
Question: Consider following gases and their respective $K_H$ values (Henry's constant) at different values of...
Consider following gases and their respective KH values (Henry's constant) at different values of temperature. In reference to these values identify correct statement/s regarding solubility of these gases in water:
| Gas | Temperature/K | KH/kbar | Gas | Temperature/K | KH/kbar |
|---|---|---|---|---|---|
| He | 293 | 144.97 | Argon | 298 | 40.3 |
| H2 | 293 | 69.16 | CO2 | 298 | 1.67 |
| N2 | 293 | 76.48 | Formaldehyde | 298 | 1.83×10−5 |
| N2 | 303 | 88.84 | |||
| O2 | 293 | 34.86 | Methane | 298 | 0.413 |
| O2 | 303 | 46.82 | Vinyl chloride | 298 | 0.611 |
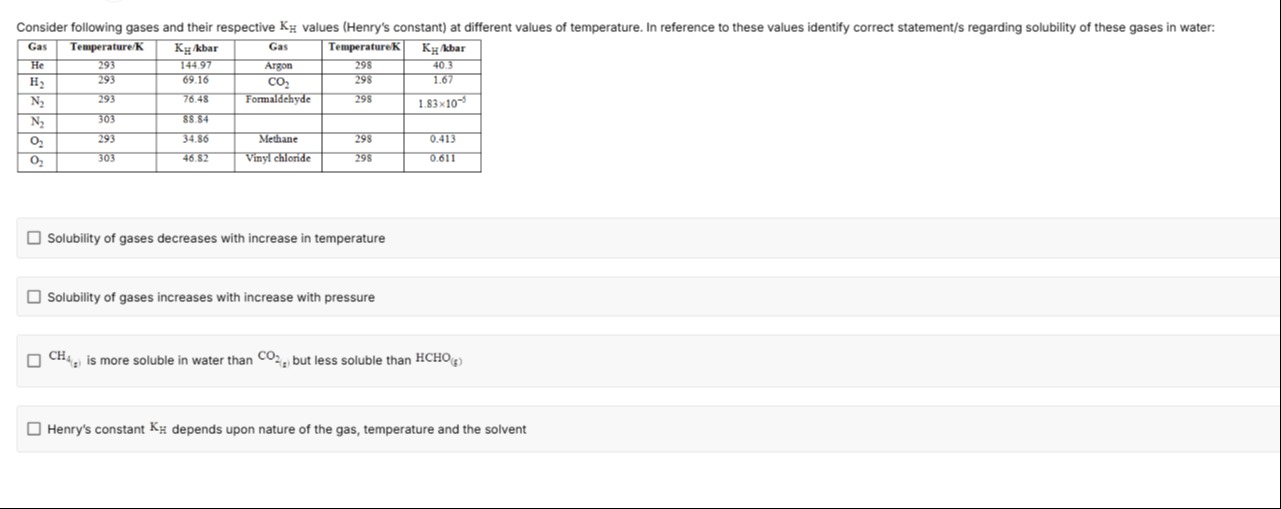
Solubility of gases decreases with increase in temperature
Solubility of gases increases with increase with pressure
CH4(g) is more soluble in water than CO2(g) but less soluble than HCHO(g)
Henry's constant KH depends upon nature of the gas, temperature and the solvent
All statements are correct.
Solution
Henry's Law states that the partial pressure of a gas in the vapor phase (p) is proportional to the mole fraction of the gas in the solution (x), given by p=KH⋅x. Solubility can be represented by the mole fraction x=p/KH. For a given partial pressure, solubility is inversely proportional to Henry's constant (KH).
Statement 1: Solubility of gases decreases with increase in temperature.
From the table, for N2, KH increases from 76.48 kbar at 293 K to 88.84 kbar at 303 K. For O2, KH increases from 34.86 kbar at 293 K to 46.82 kbar at 303 K. Since solubility is inversely proportional to KH, an increase in KH with temperature indicates a decrease in solubility with temperature. This statement is correct.
Statement 2: Solubility of gases increases with increase with pressure.
According to Henry's Law, x=p/KH. For a given temperature and gas (constant KH), the mole fraction x (solubility) is directly proportional to the partial pressure p of the gas. Therefore, increasing the pressure increases the solubility. This statement is correct.
Statement 3: CH4(g) is more soluble in water than CO2(g) but less soluble than HCHO(g).
At 298 K, the KH values are: KH(CH4)=0.413 kbar, KH(CO2)=1.67 kbar, KH(HCHO)=1.83×10−5 kbar.
Comparing the KH values: KH(HCHO)<KH(CH4)<KH(CO2).
Since solubility is inversely proportional to KH, the order of solubility is: Solubility(HCHO) > Solubility(CH4) > Solubility(CO2).
This means CH4 is more soluble than CO2 and less soluble than HCHO. This statement is correct.
Statement 4: Henry's constant KH depends upon nature of the gas, temperature and the solvent.
The table shows different KH values for different gases (e.g., He, H2, N2, O2 at 293 K), indicating dependence on the nature of the gas. The table also shows different KH values for the same gas (N2 or O2) at different temperatures, indicating dependence on temperature. Henry's constant is also known to depend on the nature of the solvent, although the table only provides data for water. This statement is correct.
All four statements are correct.
