Question
Question: Consider an electron in the $n = 3$ orbit of a hydrogen-like atom with atomic number $Z$. At absolut...
Consider an electron in the n=3 orbit of a hydrogen-like atom with atomic number Z. At absolute temperature T, a neutron having thermal energy kBT has the same de Broglie wavelength as that of this electron. If this temperature is given by T=απ2a02mNkBZ2h2, (where h is the Planck's constant, kB is the Boltzmann constant, mN is the mass of the neutron and a0 is the first Bohr radius of hydrogen atom) then the value of α is ______
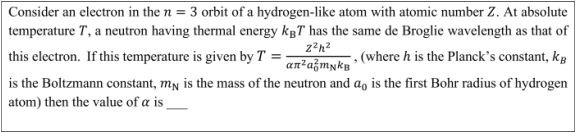
72
Solution
The de Broglie wavelength of an electron in the n-th orbit of a hydrogen-like atom with atomic number Z is given by λe=n2πrn, where rn is the radius of the n-th orbit. The radius of the n-th orbit is rn=Zn2a0, where a0 is the first Bohr radius of hydrogen.
So, the de Broglie wavelength of the electron in the n-th orbit is λe=n2π(Zn2a0)=Z2πna0.
For n=3, the de Broglie wavelength of the electron is λe=Z2π(3)a0=Z6πa0.
The de Broglie wavelength of a particle with kinetic energy E is given by λ=ph=2mEh.
The neutron has thermal energy kBT. So, the kinetic energy of the neutron is EN=kBT.
The de Broglie wavelength of the neutron is λN=2mNkBTh.
According to the problem statement, the de Broglie wavelengths are the same: λe=λN.
Z6πa0=2mNkBTh.
We are given the temperature T=απ2a02mNkBZ2h2. Substitute this expression for T into the equation:
Z6πa0=2mNkB(απ2a02mNkBZ2h2)h.
The terms mN and kB cancel out in the denominator inside the square root:
Z6πa0=απ2a022Z2h2h.
Simplify the square root in the denominator:
απ2a022Z2h2=α2π2a02Z2h2=α2πa0Zh.
So, the equation becomes:
Z6πa0=α2πa0Zhh.
Z6πa0=2Zhhαπa0.
Cancel h from the numerator and denominator on the right side:
Z6πa0=2Zαπa0.
Cancel Zπa0 from both sides:
6=2α.
Multiply both sides by 2:
α=62.
Square both sides to find α:
α=(62)2=62×(2)2=36×2=72.
The value of α is 72.
