Question
Question: Consider all possible isomeric ketones, including stereoisomers of MW=100. All these isomers are ind...
Consider all possible isomeric ketones, including stereoisomers of MW=100. All these isomers are independently reacted with NaBH4 (NOTE: stereoisomers are also reacted separately). The total number of ketones that gives racemic mixture product(s) is /are
Solution
A racemic mixture product is given by those compounds which have at least one chiral carbon atom. A chiral atom is that which is asymmetric and is attached by four different atoms or groups of atoms.
Complete answer:
To find out which ketone molecular weight is 100g, we will apply the following method.
The general formula for ketone is CnH2nO so,
The atomic weight of carbon is 12g, hydrogen is 1g and oxygen is 16g.
So to find the unknown 'n' in general formula of ketone we will do some mathematical calculation here and that is,
So the ketone with molecular weight 100g is C6H12O.
Now the different stereoisomers of the above kenotic formulae are
1. 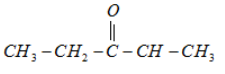
It will not form a racemic mixture product on reduction with NaBH4 because it does not have any chiral atom.
2. 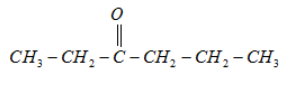
It will form a racemic mixture product on reduction with NaBH4 because its fifth carbon atom is a chiral carbon atom.
3. 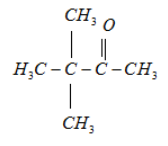
It will form racemic mixture product on reduction with NaBH4 because its fourth carbon atom is a chiral carbon atom
4. 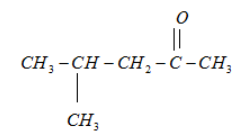
It will form racemic mixture product on reduction with NaBH4 because its third carbon atom is a chiral carbon atom
5. 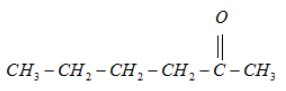
It will form a racemic mixture product on reduction with NaBH4 because its third carbon atom is a chiral carbon atom.
6. 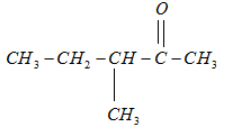
It will form a racemic mixture product on reduction with NaBH4 because its third carbon atom is a chiral carbon atom.
**Hence, there are five ketones that give a racemic mixture product.
Note:**
The students generally get confused about which chiral carbon atom. So kindly read the chiral carbon atom properties clearly. The other mistake done by students is that they do not show the organic compounds (stereoisomers). Do show them otherwise you will lose your marks.
