Question
Question: Consider a thremodynamics cycle in a PV diagram shown in the figure performed by one mole of a monoa...
Consider a thremodynamics cycle in a PV diagram shown in the figure performed by one mole of a monoatomic gas. The temperature at A is T0 and volumes are related as VB=VC=2VA. Choose the correct options :-
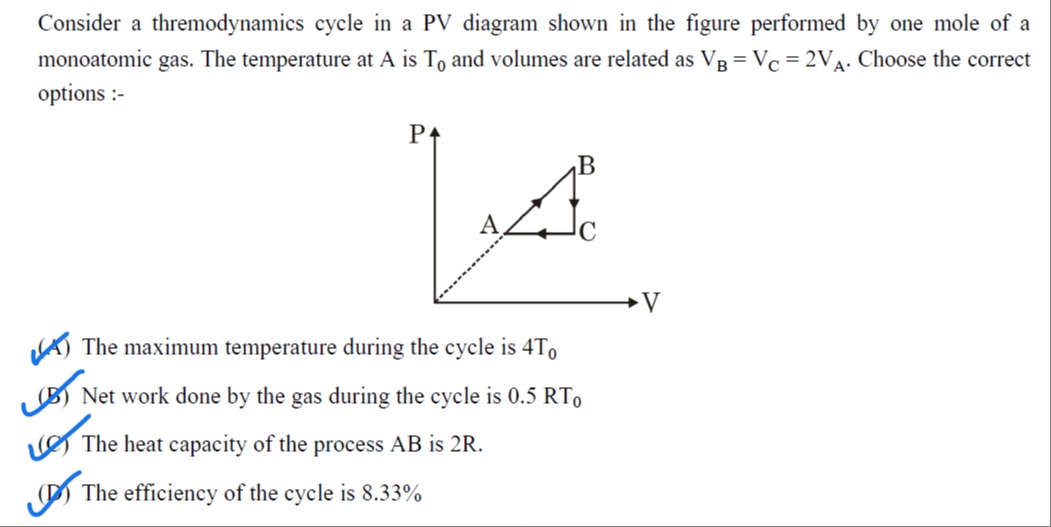
The maximum temperature during the cycle is 4T0
Net work done by the gas during the cycle is 0.5RT0
The heat capacity of the process AB is 2R.
The efficiency of the cycle is 8.33%
(A), (B), (C), (D)
Solution
Let VA=V0. Then VB=VC=2V0.
Given TA=T0. For 1 mole of ideal gas, PAVA=RTA, so PAV0=RT0, which means PA=V0RT0.
From the diagram, process C to A is isobaric, so PC=PA=V0RT0.
Process B to C is isochoric, so VB=VC=2V0.
Process A to B is a straight line in the PV diagram. From the dashed line passing through the origin and A, it is implied that the process A to B is of the form P∝V. Let P=kV.
At point A, PA=kVA, so V0RT0=kV0, which gives k=V02RT0.
So the equation for process A to B is P=V02RT0V.
At point B, VB=2V0, so PB=V02RT0(2V0)=V02RT0.
The coordinates of the points are:
A: (V0,V0RT0)
B: (2V0,V02RT0)
C: (2V0,V0RT0)
Temperatures at the points:
TA=RPAVA=R(RT0/V0)V0=T0.
TB=RPBVB=R(2RT0/V0)(2V0)=4T0.
TC=RPCVC=R(RT0/V0)(2V0)=2T0.
(A) The maximum temperature during the cycle is 4T0.
Process A to B: T=RPV=R(RT0/V02)V⋅V=V02T0V2. As V goes from V0 to 2V0, T increases from T0 to 4T0.
Process B to C: Isochoric, V=2V0. P changes from PB=2RT0/V0 to PC=RT0/V0. Temperature changes from TB=4T0 to TC=2T0. Temperature decreases linearly with pressure.
Process C to A: Isobaric, P=RT0/V0. V changes from VC=2V0 to VA=V0. Temperature changes from TC=2T0 to TA=T0. Temperature decreases linearly with volume.
The maximum temperature is 4T0 at point B. Option (A) is correct.
(B) Net work done by the gas during the cycle is 0.5RT0.
The net work done is the area enclosed by the cycle in the PV diagram. The cycle is a triangle ABC.
Wnet=Area of triangle ABC=21×base×height.
Considering the base along the vertical line V=2V0 (from C to B), the base length is PB−PC=V02RT0−V0RT0=V0RT0.
The height is the horizontal distance from the line V=2V0 to point A, which is VC−VA=2V0−V0=V0.
Wnet=21×V0RT0×V0=21RT0=0.5RT0.
Since the cycle is clockwise, the work done is positive. Option (B) is correct.
(C) The heat capacity of the process AB is 2R.
Process A to B is P=V02RT0V, which is of the form P=constant⋅V. This can be written as PV−1=constant.
For a process PVx=constant, the molar heat capacity is C=CV+1−xR. Here x=−1.
For a monoatomic gas, CV=23R.
CAB=CV+1−(−1)R=23R+2R=24R=2R.
Option (C) is correct.
(D) The efficiency of the cycle is 8.33%.
Efficiency η=QinWnet. We need to find the heat absorbed during the cycle.
Wnet=0.5RT0.
Heat absorbed in process A to B: QAB=ΔUAB+WAB.
ΔUAB=CV(TB−TA)=23R(4T0−T0)=23R(3T0)=29RT0.
WAB=∫VAVBPdV=∫V02V0V02RT0VdV=V02RT0[2V2]V02V0=V02RT0(2(2V0)2−2V02)=V02RT0(24V02−V02)=V02RT023V02=23RT0.
QAB=29RT0+23RT0=212RT0=6RT0. Since QAB>0, heat is absorbed.
Heat in process B to C: Isochoric, WBC=0. QBC=ΔUBC=CV(TC−TB)=23R(2T0−4T0)=23R(−2T0)=−3RT0. Since QBC<0, heat is released.
Heat in process C to A: Isobaric. QCA=CP(TA−TC). For monoatomic gas, CP=CV+R=23R+R=25R.
QCA=25R(T0−2T0)=25R(−T0)=−25RT0. Since QCA<0, heat is released.
Total heat absorbed Qin is the sum of positive heat transfers. In this cycle, only process A to B involves heat absorption.
Qin=QAB=6RT0.
Efficiency η=QinWnet=6RT00.5RT0=60.5=121.
As a percentage, η=121×100%=8.333...%.
Option (D) is correct.
Since multiple options are correct, we check all of them. Options (A), (B), (C), and (D) are all correct.
