Question
Question: Conjugate base of \(N{H_3}\) is: A.\(N{H_4}^ + \) B.\(N{H_2}^ + \) C.\(N{H_2}^ - \) D.\({N_2...
Conjugate base of NH3 is:
A.NH4+
B.NH2+
C.NH2−
D.N2
Solution
We can define Bronsted-Lowry acid as proton donor and comprises a hydrogen atom. It may be a neutral molecule or may contain a net positive or negative charge.
We can define a conjugate base as the product formed by a loss of proton from an acid. The conjugate base of the acid A will be A - .
Complete step by step answer:
Based on the Bronsted-Lowry Theory, a substance, which releases a proton, is an acid and a base is a substance, which accepts a proton.
Let us consider an example of hydrochloric acid reacting with ammonia to form ammonium ions and chloride ions.
We can write the chemical reaction as,
HCl(aq)+NH3(aq)NH4+(aq)+Cl−(aq)
In the above equation, we can see that hydrochloric acid has donated a proton to ammonia, and ammonia has accepted a proton. Therefore, we can say hydrochloric acid is Bronsted-Lowry acid (proton donor), and ammonia as Bronsted-Lowry base (proton acceptor). Here, ammonium ion is a conjugate acid of ammonia and chloride ions are conjugate bases of hydrochloric acid.
We can define a conjugate base as the product formed by loss of proton from an acid. The conjugate base of the acid A will be A−.
A conjugate acid is the product formed by gain of a proton by a base. The conjugate acid of the base B will be HB+.
Now let us identify the conjugate base of NH3.
Ammonia (NH3) loses a proton and acts as an acid. We can write the chemical equation as,
NH3−H+NH2−
Ammonia loses a proton and forms its conjugate base NH2−.
Ammonia is the acid and NH2− is the conjugate base of NH3.
We can give the structure of ammonia as,
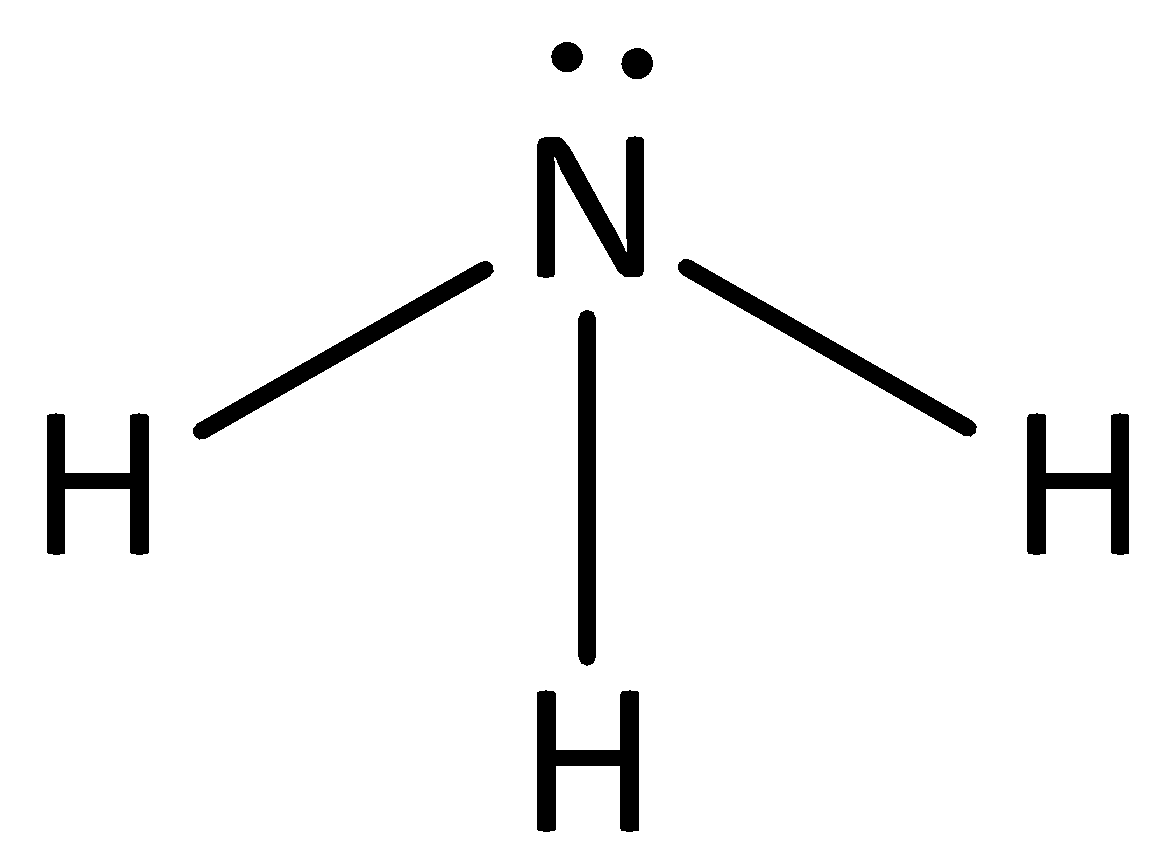
The conjugate base of ammonia will have the structure as,
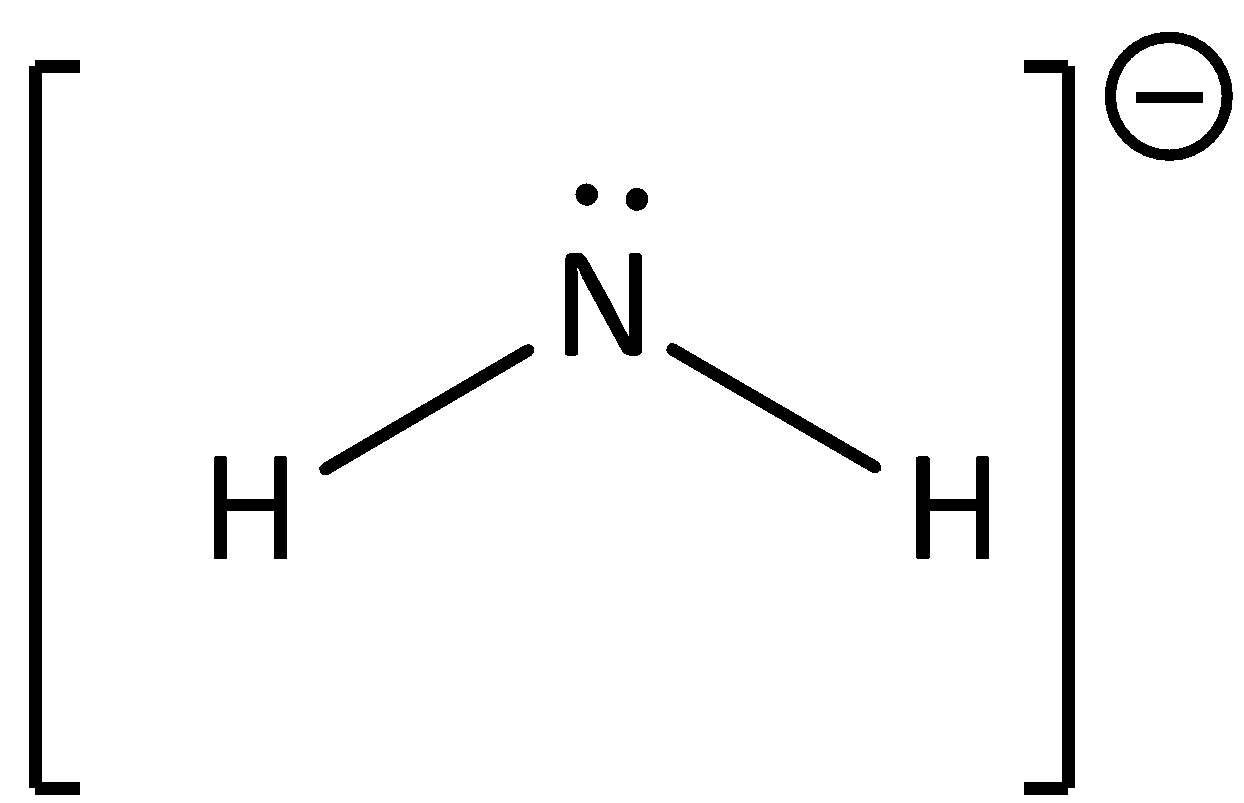
∴Option (C) is correct.
Note:
We know that acid loses a proton and forms a conjugate base. Base accepts a proton and forms conjugate acid.
Example 1: Let us consider the reaction given below,
NH3(g)+HI(g)I−(aq)+NH4+(aq)
Hydrogen iodide loses its proton to form iodide. Ammonia gains a proton to form ammonium ion.
The acid in the reaction is HI
The conjugate base of the acid is I−
The base in the reaction is NH3
The conjugate acid of the base is NH4+
Example 2: Let us consider the reaction given below,
HCOOH(l)+H2O(l)HCOO−(aq)+H3O+(aq)
Formic acid loses a proton to form formate ion. Water gains a proton and forms hydronium ion.
The acid in the reaction is HCOOH
The conjugate base of the acid is HCOO−
The base in the reaction is H2O
The conjugate acid of the base is H3O+
Example 3: Let us consider the reaction given below,
HSO4(aq)−+H2O(l)H2SO4(aq)+OH−(aq)
HSO4− gains a proton and forms sulphuric acid. Water loses a proton to form hydronium ion.
The acid in the reaction is H2O
The conjugate base of the acid is OH−
The base in the reaction is HSO4−
The conjugate acid of the base is H2SO4
