Question
Question: Compute the enthalpy of formation of liquid methyl alcohol in kJ mol-¹, using the following data. E...
Compute the enthalpy of formation of liquid methyl alcohol in kJ mol-¹, using the following data.
Enthalpy of vaporisation of liquid CH3OH = 38 kJ/mol.
Enthalpy of formation of gaseous atoms from the elements in their standard states are H→218 kJ/mol; C→715 kJ/mol; O→249 kJ/mol.
Bond Enthalpies C-H→415 kJ/mol; C-0→356 kJ/mol; O-H→463 kJ/mol
-266 kJ mol⁻¹
Solution
To compute the enthalpy of formation of liquid methyl alcohol (CH3OH), we can use Hess's Law by breaking down the overall formation process into several steps involving atomization and bond formation.
The target reaction is: C(graphite)+2H2(g)+21O2(g)→CH3OH(l)
We can follow these steps:
Step 1: Atomization of reactants
Convert the elements in their standard states to gaseous atoms. C(graphite)→C(g); ΔHa(C)=715 kJ/mol 2H2(g)→4H(g); Since H2(g)→2H(g) requires 2×218 kJ/mol, for 2H2(g) it will be 2×(2×218)=4×218=872 kJ/mol. 21O2(g)→O(g); ΔHa(O)=249 kJ/mol
The total enthalpy change for atomization of reactants (ΔH1) is: ΔH1=ΔHa(C)+4×ΔHa(H)+ΔHa(O) ΔH1=715 kJ/mol+872 kJ/mol+249 kJ/mol ΔH1=1836 kJ/mol
Step 2: Formation of gaseous methyl alcohol from gaseous atoms
This step involves the formation of bonds. The structure of CH3OH is:
H
|
H-C-O-H
|
H
It contains:
- 3 C-H bonds
- 1 C-O bond
- 1 O-H bond
When bonds are formed, energy is released (exothermic, negative enthalpy change). Enthalpy change for bond formation (ΔH2): ΔH2=−[3×BE(C-H)+1×BE(C-O)+1×BE(O-H)] ΔH2=−[3×415 kJ/mol+1×356 kJ/mol+1×463 kJ/mol] ΔH2=−[1245+356+463] kJ/mol ΔH2=−2064 kJ/mol
The enthalpy of formation of gaseous methyl alcohol (ΔHf∘(CH3OH(g))) from its elements is the sum of ΔH1 and ΔH2: ΔHf∘(CH3OH(g))=ΔH1+ΔH2=1836 kJ/mol+(−2064 kJ/mol) ΔHf∘(CH3OH(g))=−228 kJ/mol
Step 3: Condensation of gaseous methyl alcohol to liquid methyl alcohol
This is the reverse of vaporization. CH3OH(g)→CH3OH(l) The enthalpy change for this step (ΔH3) is the negative of the enthalpy of vaporization: ΔH3=−ΔHvap(CH3OH(l))=−38 kJ/mol
Overall Enthalpy of Formation of Liquid Methyl Alcohol
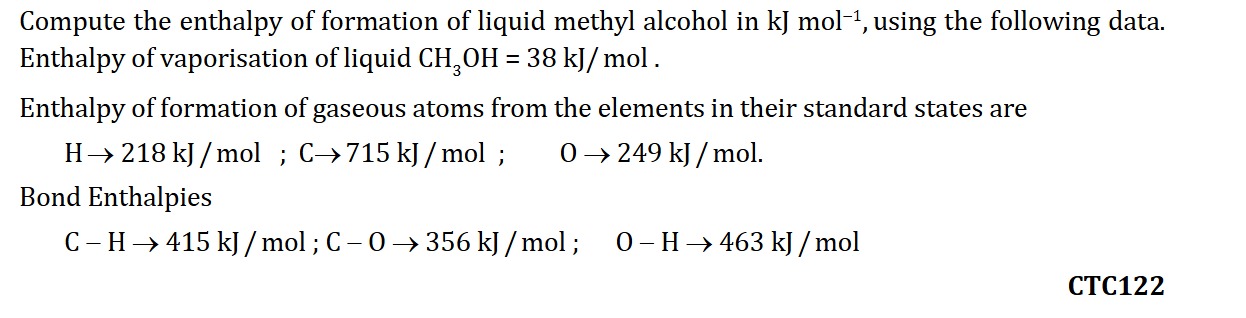
The total enthalpy of formation of liquid methyl alcohol (ΔHf∘(CH3OH(l))) is the sum of the enthalpy changes of all steps: ΔHf∘(CH3OH(l))=ΔHf∘(CH3OH(g))+ΔH3 ΔHf∘(CH3OH(l))=−228 kJ/mol+(−38 kJ/mol) ΔHf∘(CH3OH(l))=−266 kJ/mol
The final answer is −266 kJ mol−1.
Explanation of the solution:
- Calculate the energy required to convert elements in their standard states to gaseous atoms (atomization energy). ΔHatomization=ΔHf(C(g))+4×ΔHf(H(g))+ΔHf(O(g))=715+4(218)+249=1836 kJ/mol.
- Calculate the energy released when gaseous atoms form gaseous methyl alcohol by forming bonds. ΔHbond formation=−(3×BE(C-H)+1×BE(C-O)+1×BE(O-H))=−(3×415+356+463)=−2064 kJ/mol.
- The enthalpy of formation of gaseous methyl alcohol is the sum of these two steps: ΔHf(CH3OH(g))=1836−2064=−228 kJ/mol.
- Finally, convert gaseous methyl alcohol to liquid methyl alcohol using the enthalpy of vaporization. Since we are going from gas to liquid, it's the negative of vaporization enthalpy. ΔHcondensation=−ΔHvaporization=−38 kJ/mol.
- The enthalpy of formation of liquid methyl alcohol is the sum of the enthalpy of formation of gaseous methyl alcohol and the enthalpy of condensation: ΔHf(CH3OH(l))=−228−38=−266 kJ/mol.
Answer: −266 kJ mol−1
