Question
Question: Compounds Such as alcohols and glucose also contain hydrogen, but are not categorized as acids. Desc...
Compounds Such as alcohols and glucose also contain hydrogen, but are not categorized as acids. Describe an activity to prove it.
Solution
The compounds which can conduct electricity in aqueous solution are acids. The compounds that cannot conduct electricity in aqueous solution do not act as acids. The acids can dissociate in aqueous solution to produce ions specifically hydronium ions. The ions are responsible for conduction of electricity.
Complete step-by-step answer: “Acids are the compounds which form the ions in the aqueous solution.” For the understanding of the acids we have to perform the activity to prove that all hydrogen containing compounds are not acid.
Activity: Let’s take one beaker containing the solution of glucose in water. In the beaker put one rubber cork and stand two nails on it which is connected to the wire of the battery. That battery is connected to the one bulb and switch.
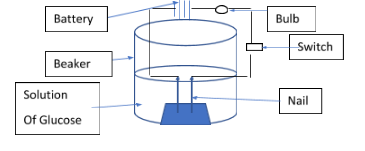
Now, as shown in the above diagram, make it set up for activity. So, now switch on the key that is connected to the battery and one end will connect to the nail. Now in case the Glucose bulb doesn’t glow, this clearly indicates that there is no formation of hydrogen cation (H+) . Since cation is not formed there is no flow of ions in the solution. Electricity passes through the solution if and only if the solution contains the ions in it. Hence, we can say that glucose is not an acid. Because it does not form ions in the solution. Similarly, we can perform activity for alcohol too, that also do not form ions hence, not categorized as the acid.
Hence, from the above activity we can say that glucose and alcohol is not categorized as acids because, in solution they cannot form the hydrogen ion.
Note: From the above activity we can conclude that the compounds such as glucose and alcohols in its aqueous solution cannot conduct electricity and hence, they are not considered as acid though they have hydroxyl groups present in it. They are covalent compounds which are soluble in water due to the presence of Hydrogen bonding and not ionize.
