Question
Question: Compound (X) with molecular formula \({C}_{3}{H}_{8}O\) is treated with acidified potassium dichroma...
Compound (X) with molecular formula C3H8O is treated with acidified potassium dichromate to form a product (Y) with molecular formula C3H6O. (Y) does not form a shining silver mirror on warming with ammoniacal AgNO3. (Y) when treated with an aqueous solution of NH2CONHNH2, HCl and sodium acetate, gives a product (Z). The structure of (Z) is:
A. CH3CH2CH=NNHCONH2
B. (CH3)2=NNHCONH2
C. (CH3)2=NCONHNH2
D. CH3CH2CH=NCONHNH2
Solution
The Tollen's reagent test is used to distinguish between aldehyde and a ketone. If after the reaction a silver mirror is formed then it indicates the presence of a ketone group, else an aldehyde group is present.
Complete step by step answer: It is given in the question that a compound X that has a molecular formula C3H8O is treated with acidified potassium dichromate to form a product (Y) with molecular formula C3H6O. The reaction can be written as follows.
(C3H8O)XH+K2Cr2O7(C3H6O)Y
And then the compound Y, when heated with ammoniacal AgNO3 does not give a shining silver mirror. After this the compound Y is made to react with an aqueous solution of NH2CONHNH2, HCl and sodium acetate, gives a product (Z). The reaction can be written as follows.
(C3H6O)YAq.(NH2CONHNH2+HCl+CH3COO−Na+)Z
Let us now find out the compounds X, Y, and Z.
We know that aldehydes gives a shining silver mirror but in this case no shining silver mirror is formed. Therefore, from this we can come to the conclusion that the compound Y is a ketone. Thus, the molecular structure of compound Y is given as:
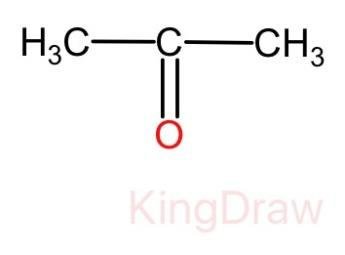
And we know that the compound X is getting oxidized with the help of potassium dichromate. Therefore, the compound X must be alcohol. Thus, the molecular structure of compound X is given as:
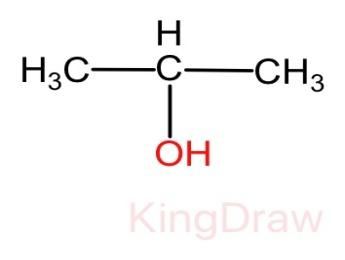
Now the reaction of compound X to form compound Y when treated with acidic potassium dichromate can be given as follows.
Propan−2−olCH3−CH(OH)−CH3H+K2Cr2O7PropanoneCH3−CO−CH3
And further, when the ketone i.e., propanone is reacted with an aqueous solution of NH2CONHNH2, HCl and sodium acetate, the oxygen in the compound Y gets replaced by NH2NHCONH2. The reaction involved is given as:
PropanoneCH3−CO−CH3Aq.(NH2CONHNH2+HCl+CH3COO−Na+)(CH3)2=NNHCONH2
Therefore, the compound Z is (CH3)2=NNHCONH2. Hence, option (B) is the correct option.
Note: The −NH2 group is an electron withdrawing group and shows -I effect. And the more the no. of EWG, the more the electron density at the atom and thus more reactive. Therefore, NH2NHCONH2 gets attached from the side which has more no. of −NH2 groups.
