Question
Question: Compound (s) that on hydrogenation products optically inactive compounds (s) is (are): (A)  that on hydrogenation products optically inactive compounds (s) is (are):
(A) 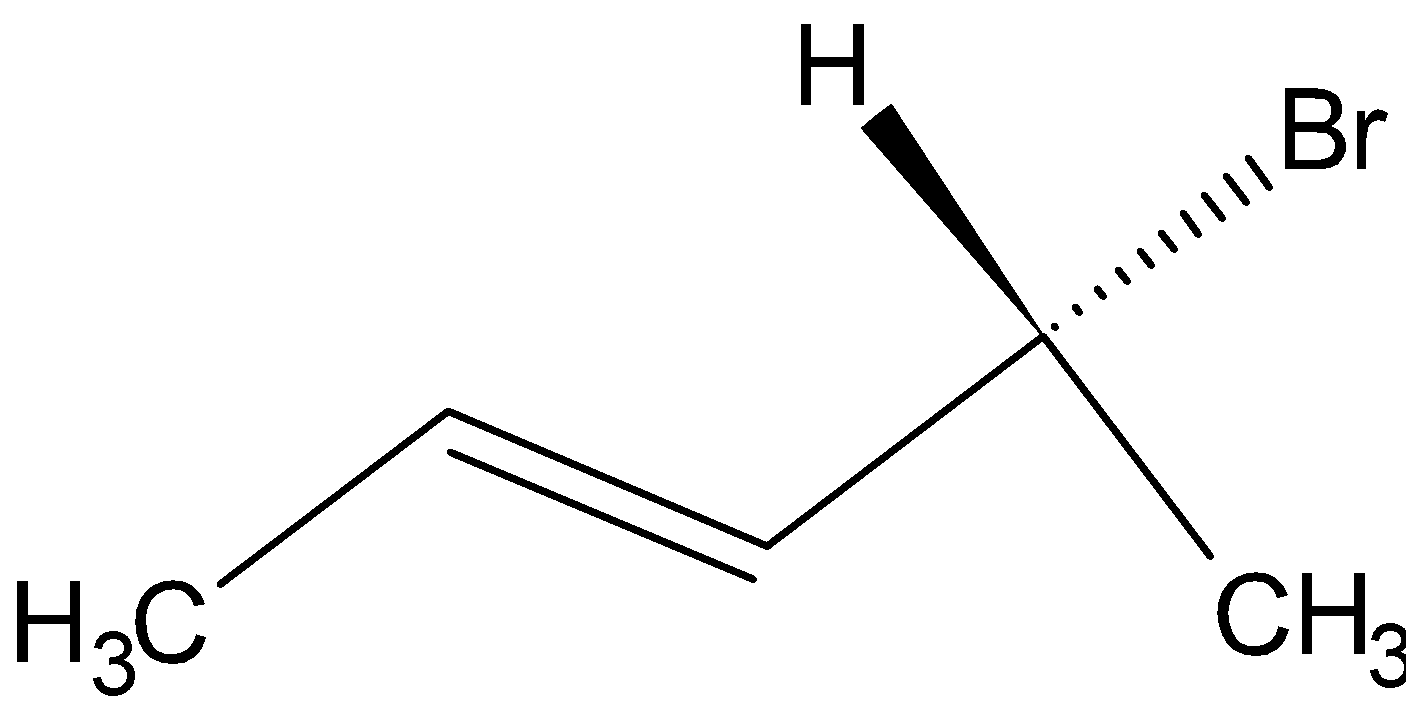
(B) 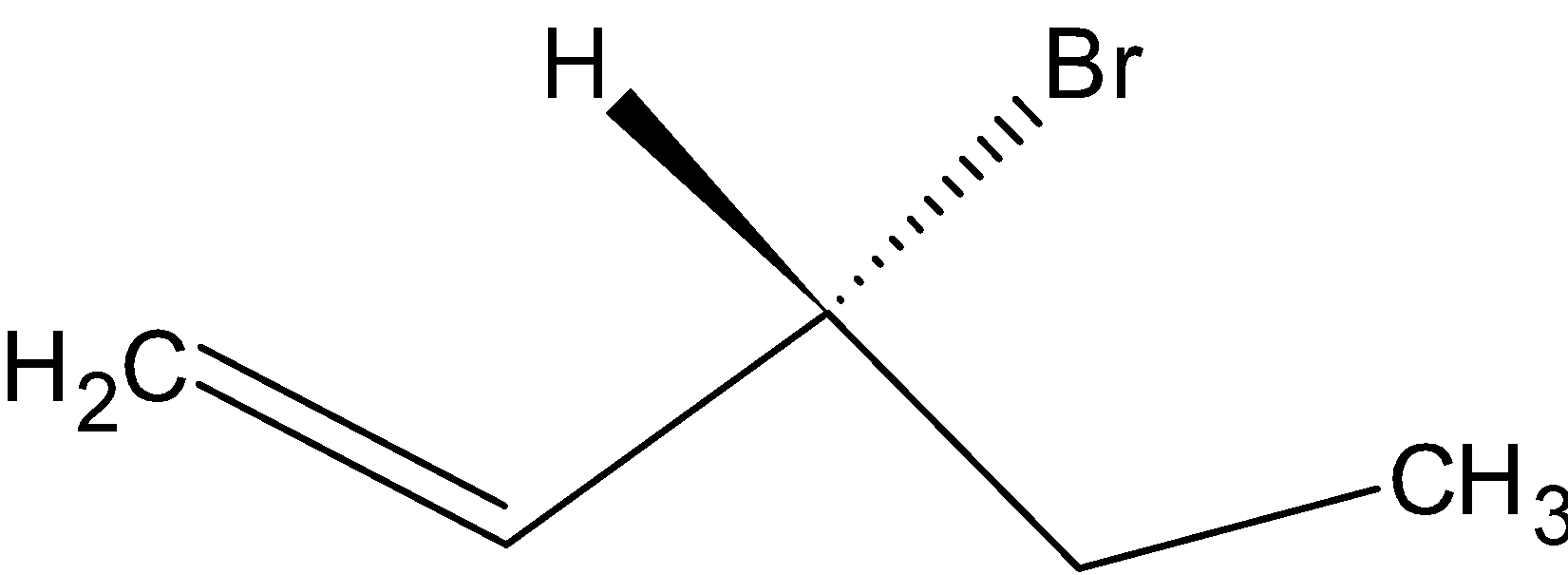
(C) 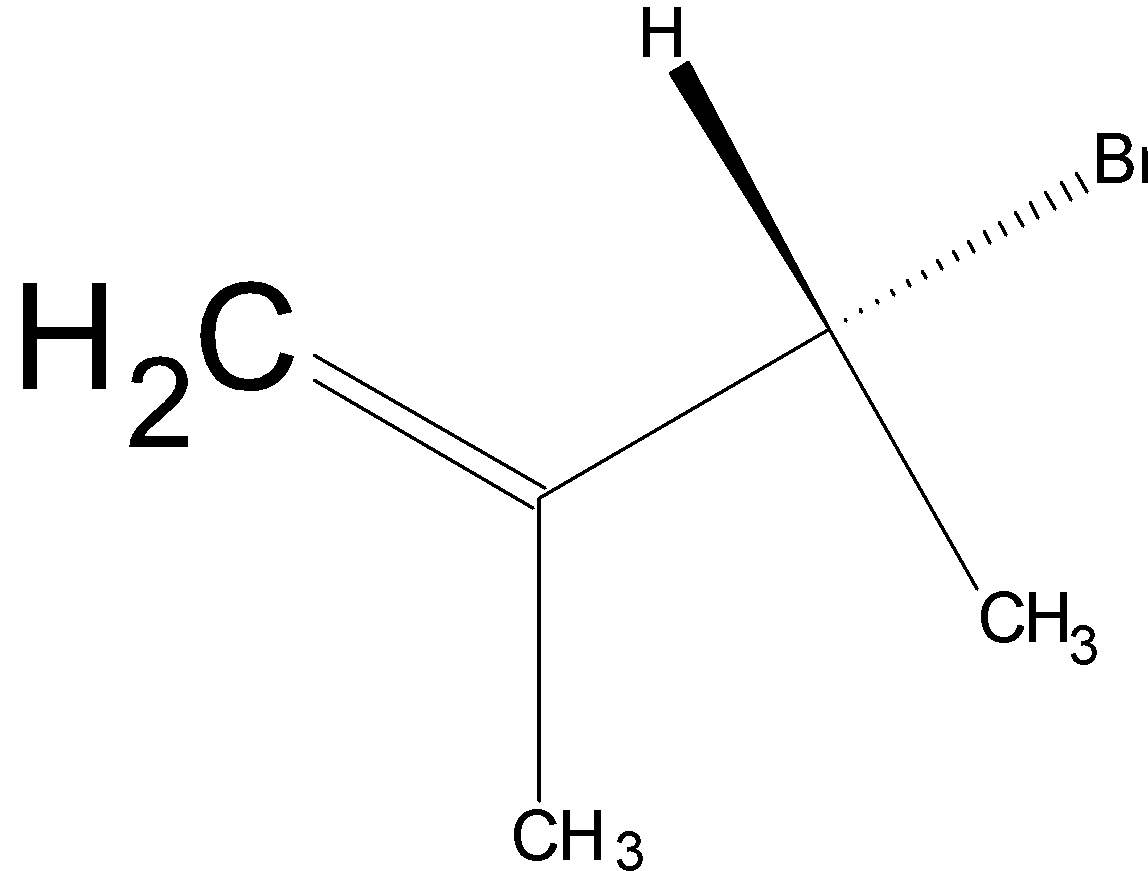
(D) 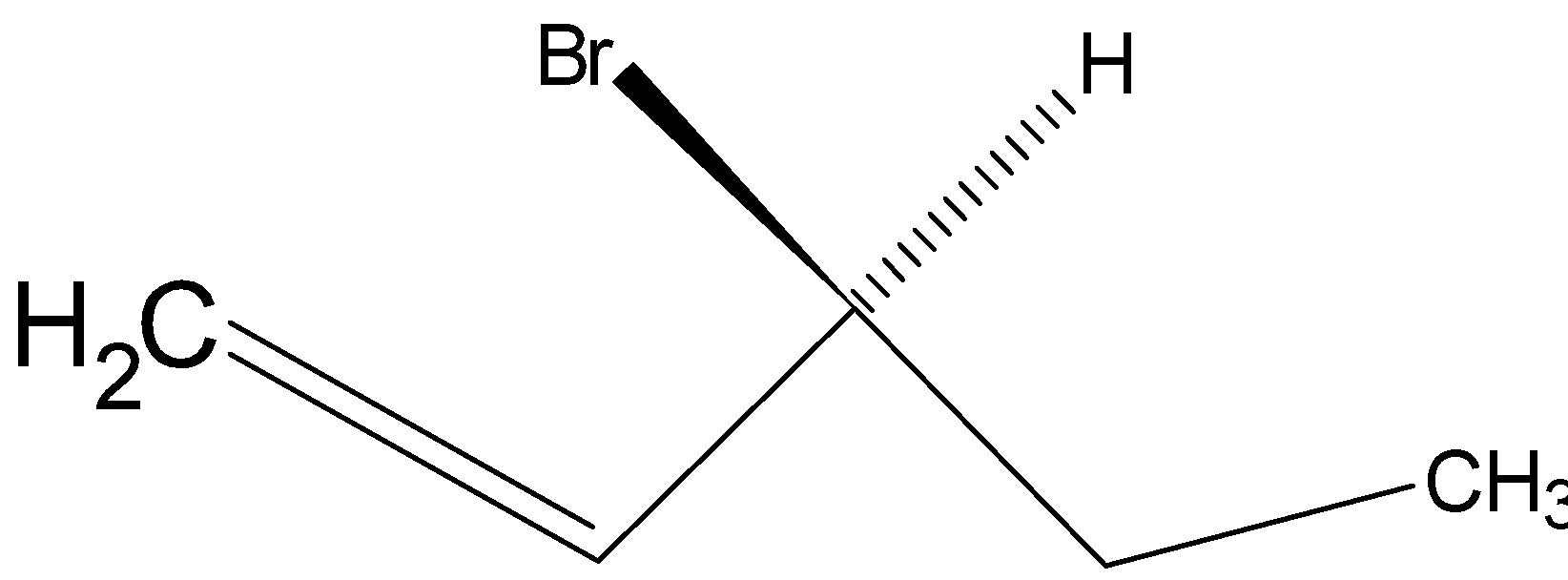
Solution
. To solve this question we should know what hydrogenation is and when a compound is said to be optically active or optically inactive. It is very important to be aware of hydrogenation of alkene.
Complete step by step answer: The process of addition of hydrogen molecule to any compound is known as hydrogenation. On hydrogenation of alkene the double is reduced and alkane is produced as the product.
A compound is said to be optically active, when a compound has a chiral carbon because the compounds with chiral carbon can rotate the plane polarised light. The optically inactive compounds are the compounds which do not have a chiral carbon in the first place, hence cannot rotate the plane polarised light.
Option A is 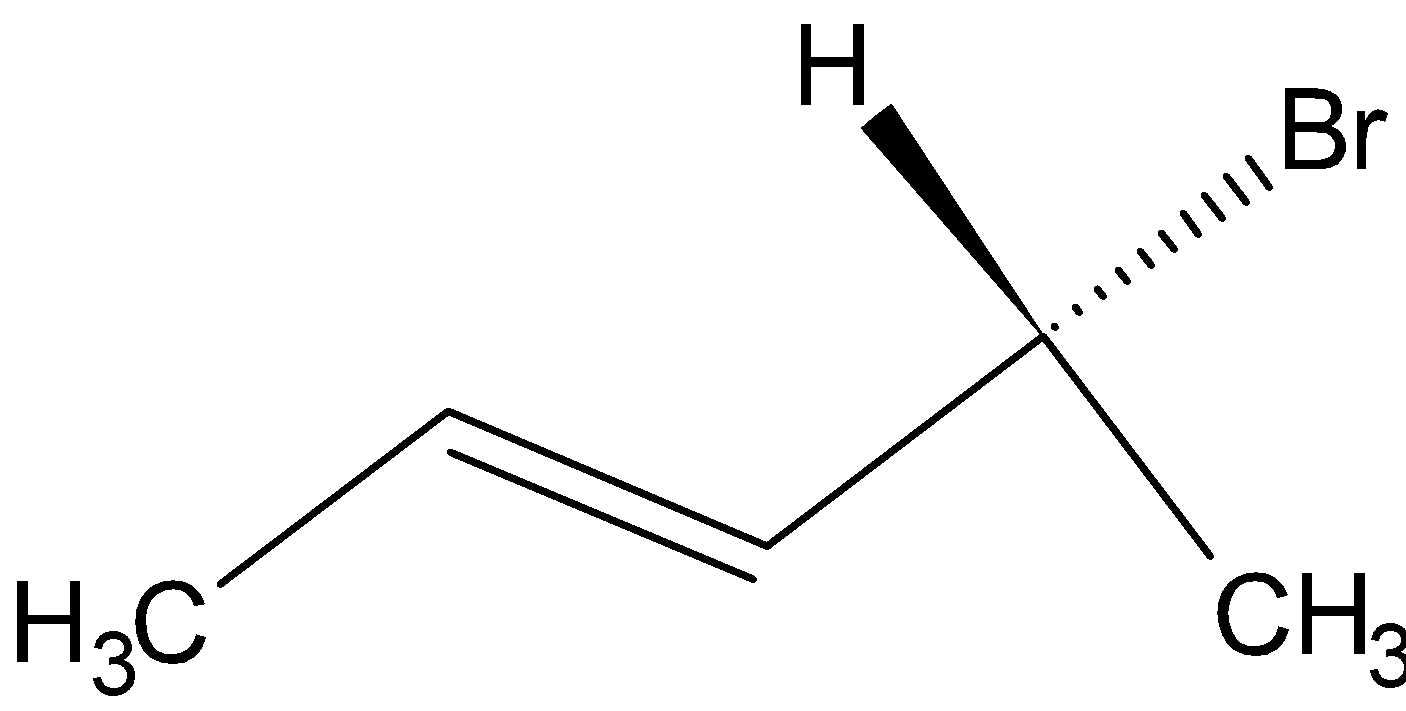 .
.
On hydrogenation, H2 reduces the double bond to single bond:
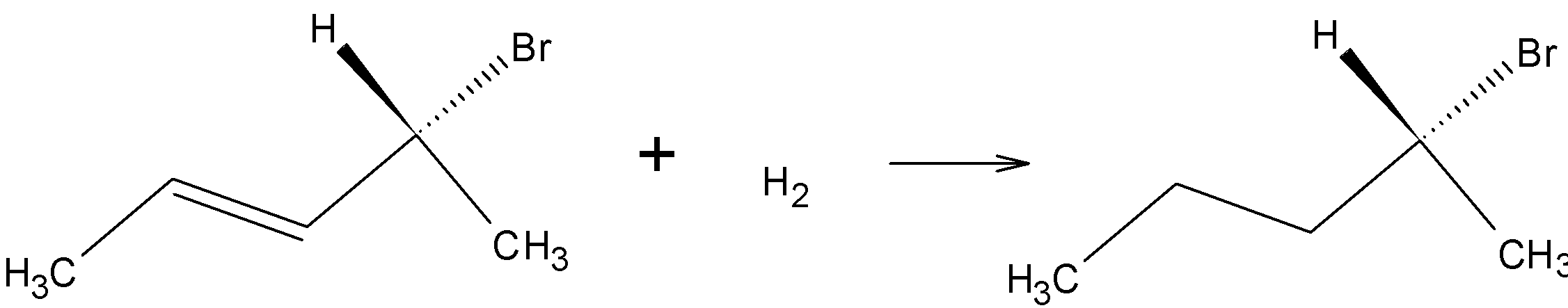
Drawing the product in Fischer projection:
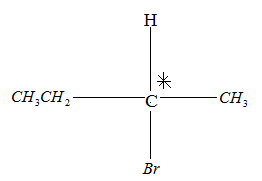
Since the compound has a chiral carbon this compound is optically active. Therefore, Option A is the wrong answer.
Option B is 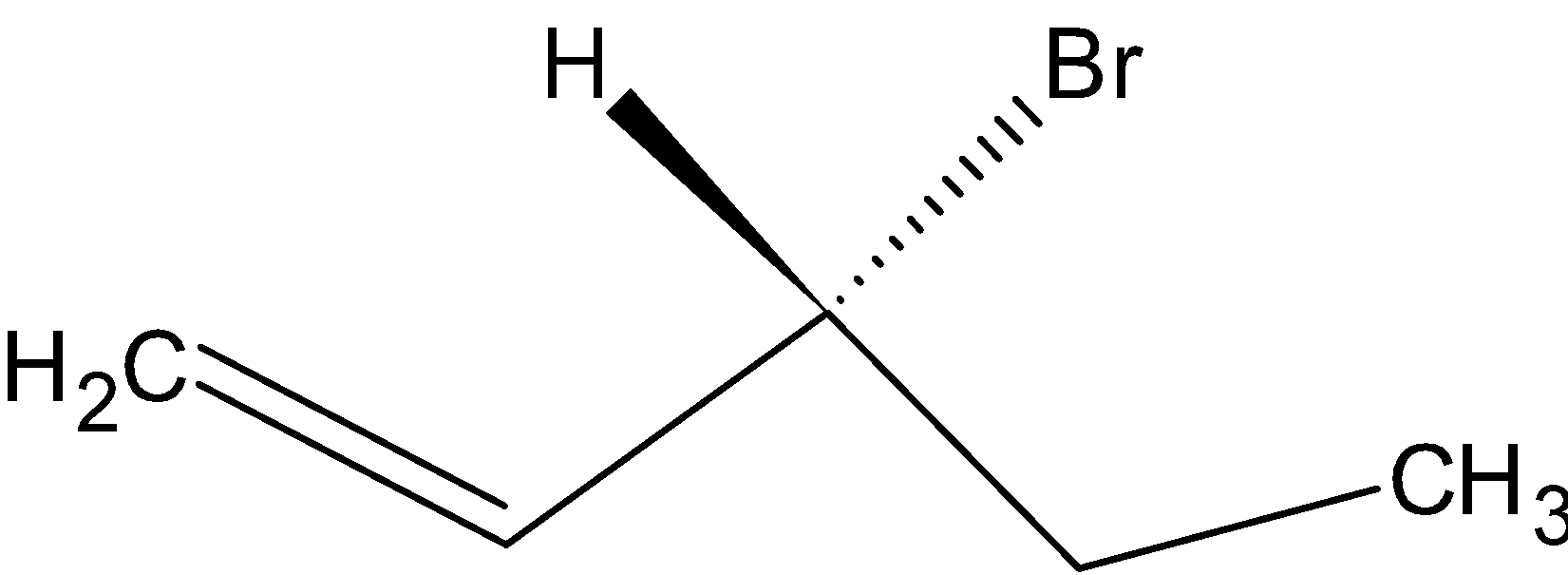 .
.
On hydrogenation, H2 reduces the double bond to single bond:
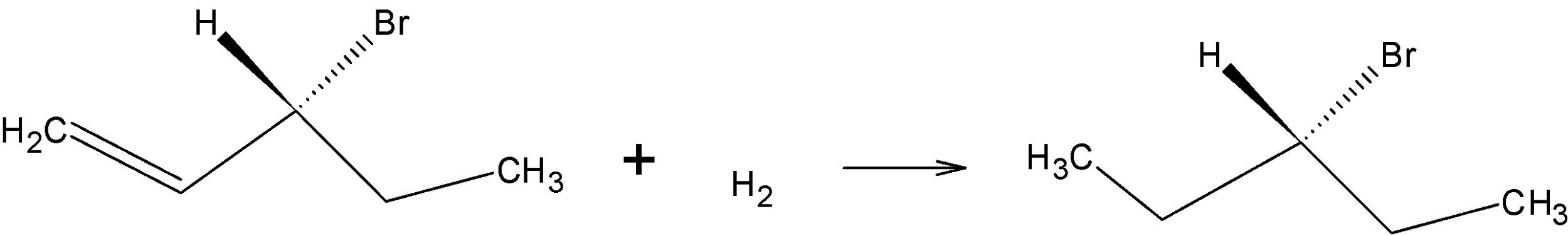
Drawing the product in Fischer projection:
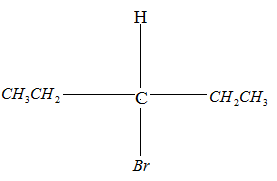
Since the compound has no chiral carbon this compound is optically inactive. Therefore, Option B is the correct answer.
Option C is 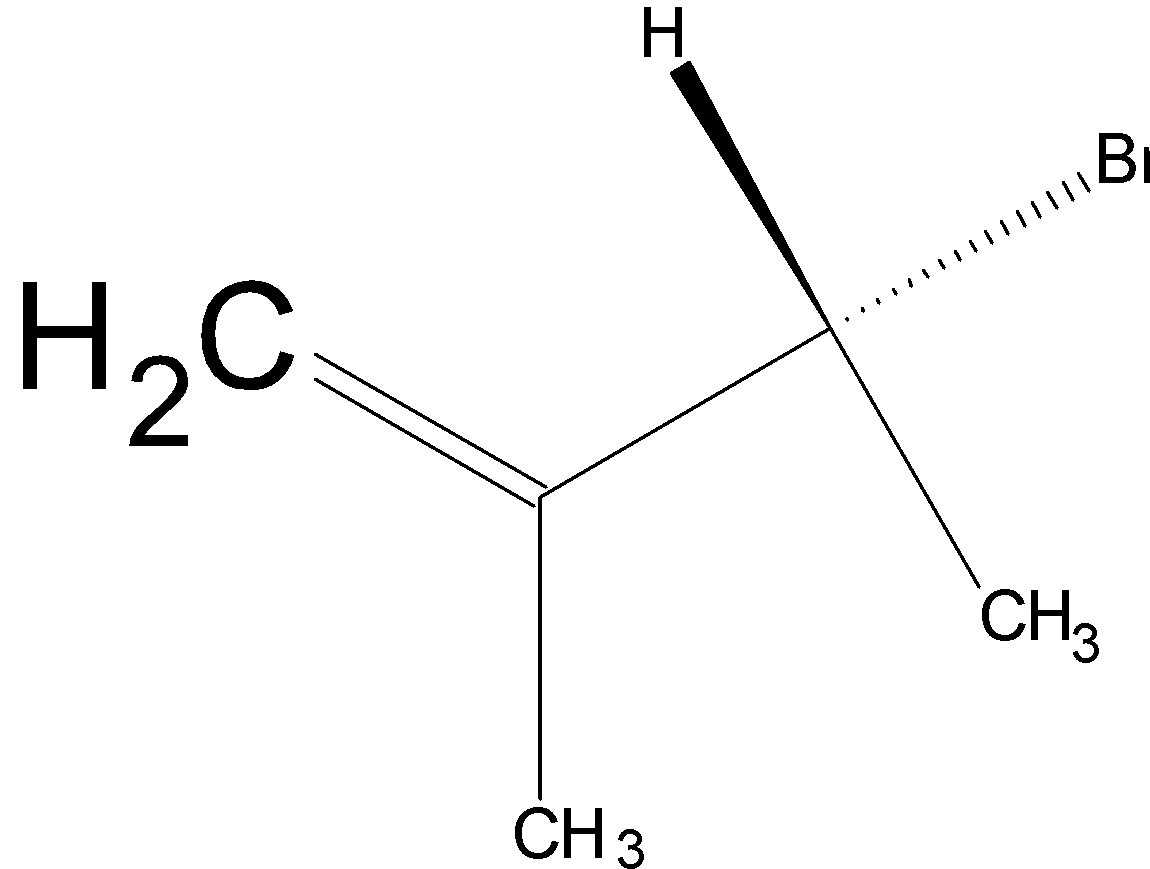 .
.
On hydrogenation, H2 reduces the double bond to single bond:
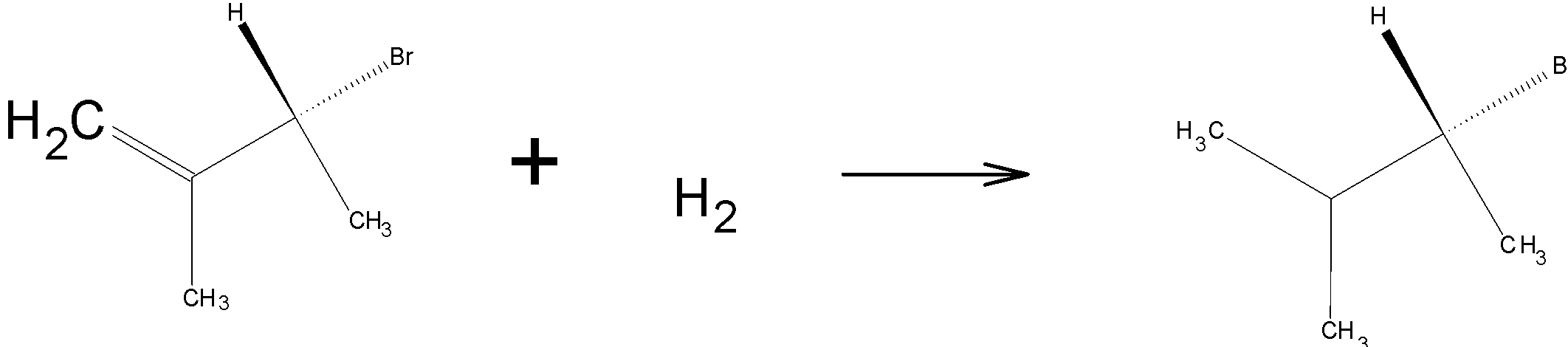
Drawing the product in Fischer projection:
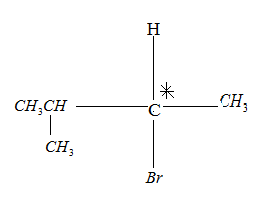
Since the compound has a chiral carbon this compound is optically active. Therefore, Option a c is the wrong answer.
Option D is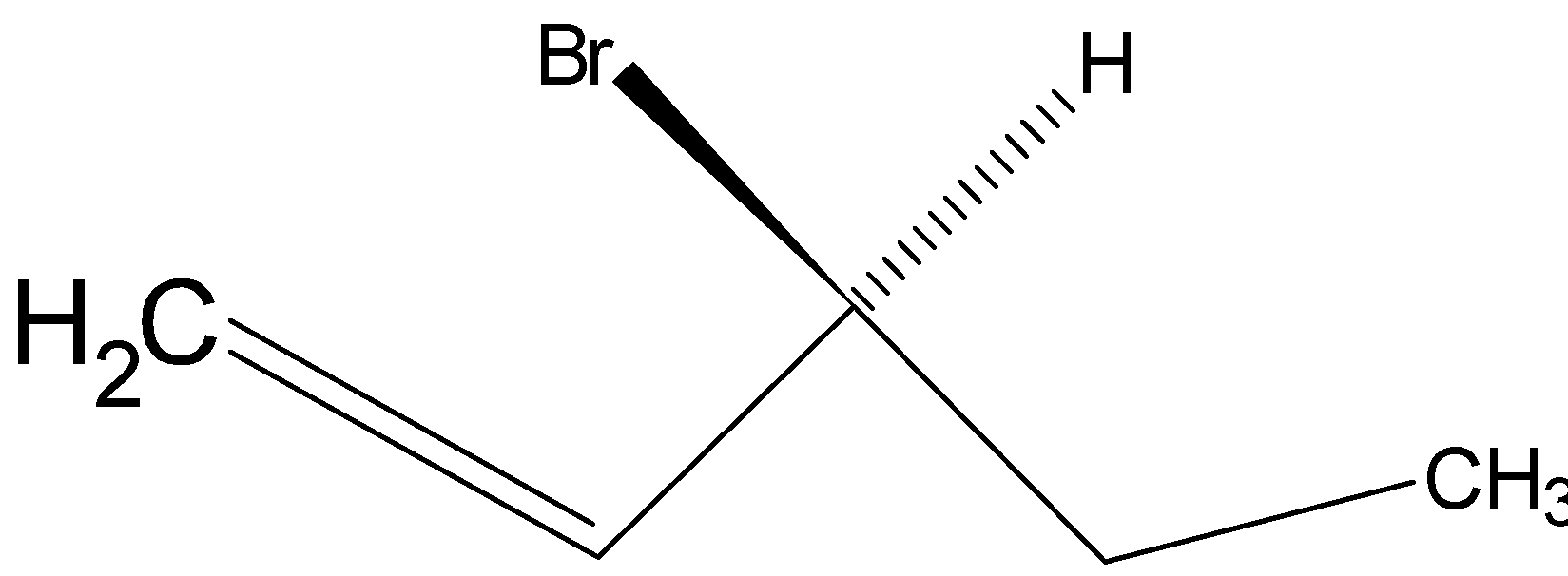 .
.
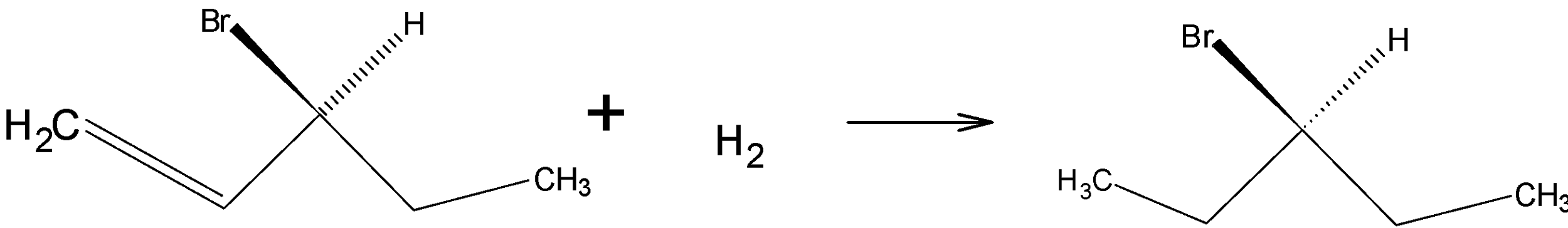
Drawing the product in Fischer projection:
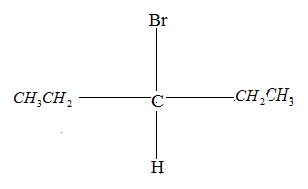
So, the correct answer is “Option B and D”.
Note: When four different groups are attached to a carbon atom, then the carbon atom is said to be chiral carbon. A compound can be optically active only when it has a chiral carbon. Always remember alkanes are formed on hydrogenation of alkenes. Drawing the product in Fischer projection will give better clarity on compounds.
