Question
Question: Compound heated with soda lime to obtain \({C_2}{H_6}\) in the laboratory is: A. \(C{H_3}C{H_2}COO...
Compound heated with soda lime to obtain C2H6 in the laboratory is:
A. CH3CH2COOH
B. CH3CH2CHO
C. CH3CH2COCH3
D. CH3CH2COONa
Solution
We must understand the formation of hydrocarbons by the decarboxylation of carboxylic acid salts by heating them with soda lime. In the decarboxylation reaction, the removal of −COOH (or) −COONa takes place and it is replaced by a hydrogen atom.
Complete step by step answer: Let’s start by discussing what is carboxylic acid? Carboxylic acids are organic aids which has the form of RCOOH, here R can be a hydrogen atom (or) alkyl group (hydrocarbon atom).The general structure of carboxylic acid is,
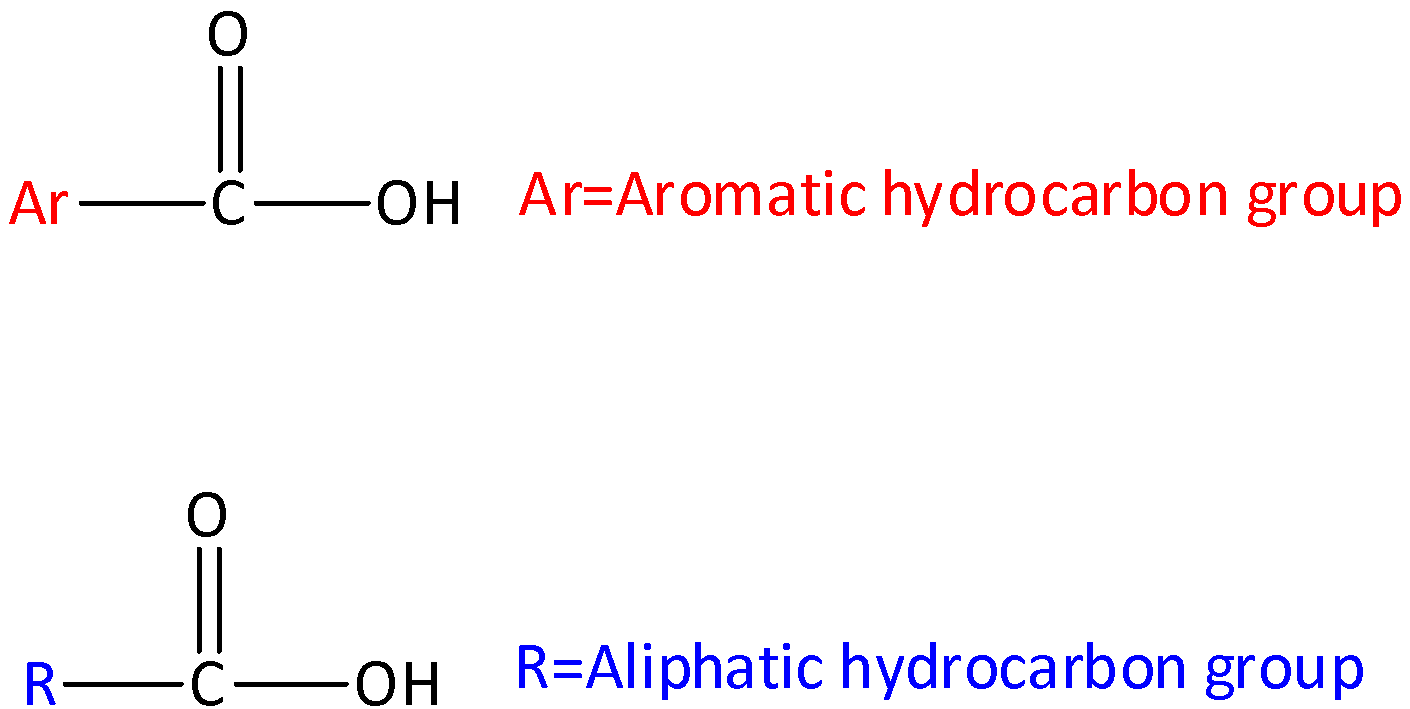
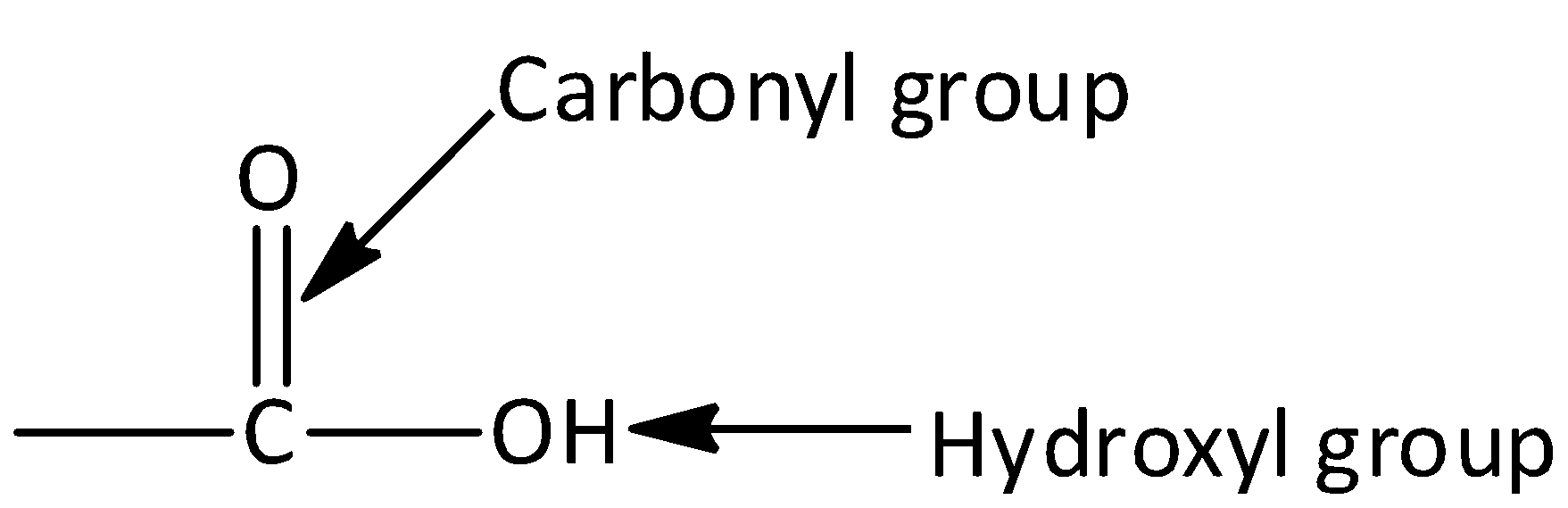
Carboxylic acid consists of two polar functional groups namely the carbonyl group and the hydroxyl group. The carbonyl group and hydroxyl group are collectively called carboxyl groups.
Now coming back, we have to identify the reactant that produces ethane gas on reacting with soda lime. Soda lime is a mixture of sodium hydroxide, calcium oxide and calcium hydroxide. It appears as white granules
We know that CH3CH2CHO is an aldehyde and therefore, it can be eliminated. And CH3CH2COCH3 is a ketone, it can also be opted out.
Now we have the remaining two options that is CH3CH2COOH and CH3CH2COONa.
Let us now understand decarboxylation. The elimination of carbon dioxide from a molecule is called decarboxylation. Sodium or potassium salts of carboxylic acid in the presence of soda lime undergo decarboxylation. The products formed are an alkane and sodium carbonate. The solid sodium salt of carboxylic acid is reacted with soda lime, to form the gas. The general reaction is,
R−COONa+NaOHCaORH+Na2CO3
Therefore, sodium propanoate will be a compound that would react with soda lime to give ethane gas in the laboratory.
CH3CH2COONa+NaOHCaOC2H6+Na2CO3 SodiumSodaEthaneSodium propanoatelimeCarbonate
Hence, option (D) is correct.
Note: We must know that the decarboxylation is also known as Duma reaction. The product alkane formed will have one carbon less than parent carboxylic acid. Aromatic carboxylic acid also undergoes this reaction.
