Question
Question: Compound D is, 
A. H2C=CH−COOH
B. 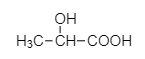
C. 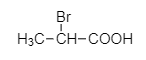
D. CH≡C−COOH
Solution
The reaction of carboxylic acid with phosphorus and bromine leads to the bromination of α carbon atom of the carboxylic acid, producing a dibromo product. The reaction is known as Hell-Volhard-Zelinsky reduction reaction. The reaction is used for the halogenation of carboxylic acid. To initiate this reaction, a catalytic amount of phosphorus tribromide is used and then bromine is added for further reaction.
Complete step by step answer:
Here, we have to find the structure of compound (D) in the following chemical reaction.
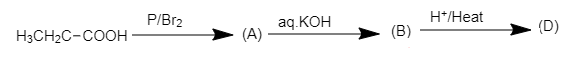
When propionic acid is treated with a catalytic amount of phosphorus and bromine, α−H substitution takes place, producing dibromo product. The substitution of α−H of propionic acid by bromine takes place on action of bromine in the presence of phosphorus. The α−H atoms of propionic acid are substituted by bromine atoms.
The chemical reaction of formation of (A) is shown below.
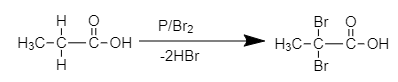
2,2-dibromo propionic acid
The product (A) is 2, 2 –dibromo propionic acid.
When the dibromo derivative of propionic acid is treated with aqueous KOH, the bromine atoms in the acid will be replaced by hydroxyl groups which on acidification will yield propionic acid with a double bond.
The complete reaction is shown below.
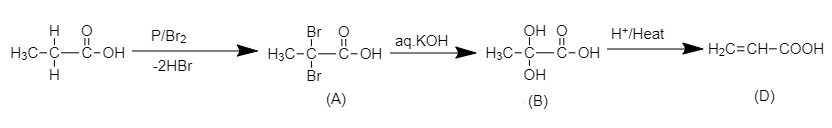
Here, compound (D) is acrylic acid and the structure of the compound is shown below.
H2C=CH−COOH
So, the correct answer is Option A.
Additional information:
Here, the dibromo derivative of propionic acid that is compound (A) is formed by Hell-Volhard-Zelinsky reduction reaction. The reaction is used for introducing halogen such as chlorine and bromine in carboxylic acid. This reaction is not applicable in case of introducing iodine and fluorine in carboxylic acid. The Hell-Volhard-Zelinsky reduction reaction.
Note: Students should keep in mind that the action of alcoholic KOH and aqueous KOH on carboxylic acid is different. The use of aqueous KOH is done in case of the chemical reactions which are not water sensitive and used for carrying out the hydrolysis reaction. The alcoholic KOH is used in case of water sensitive reaction and to carry out dehydration of the compound.
