Question
Question: Compound \({C_4}{H_8}{O_2}\) exits in various structures as shown: Which statement(s) is/are corre...
Compound C4H8O2 exits in various structures as shown:
Which statement(s) is/are correct?
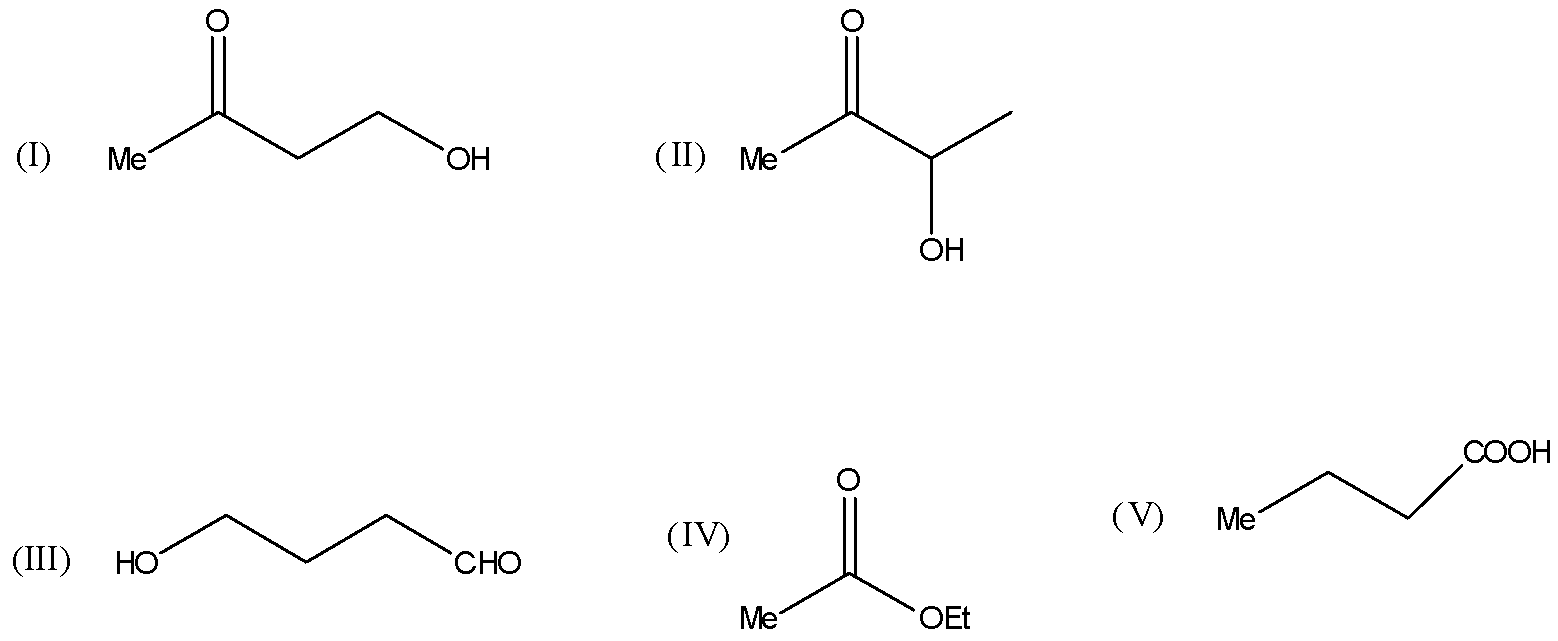
A) Compounds I and II give iodoform test: Compound I gives turbidity on heating with Lucas reagent, while compound II reduces Tollens’ reagent.
B) Compound III gives a silver mirror with [Ag(NH3)2]⊕ and does not react with NaOBr.
C) Compound IV on acid hydrolysis gives C2H5COOH and MeOH.
D) Compound V on heating is decarboxylated to propane.
Solution
Tollens’ test is given by all aldehydes and α- hydroxy ketones. All alcohol shows turbidity with Lucas reagent. Iodoform test is given by the compounds having methyl ketone groups. Also, you should know that only β- keto acids undergo decarboxylation on heating.
Complete step by step solution:
In the question we are given a compound having molecular formula, and its various structures are given. Let us discuss the statements given in options one by one:
A) Compounds I and II give iodoform test: Compound I gives turbidity on heating with Lucas reagent, while compound II reduces Tollens’ reagent.
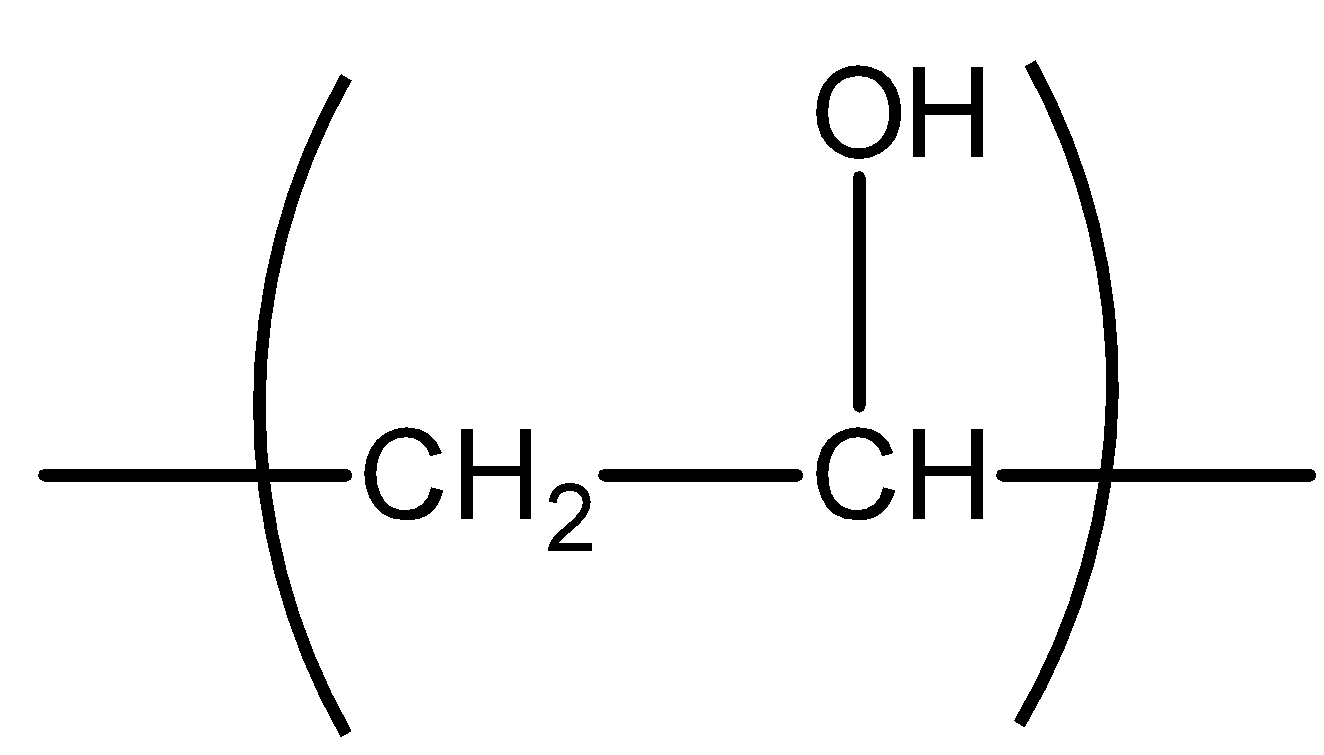
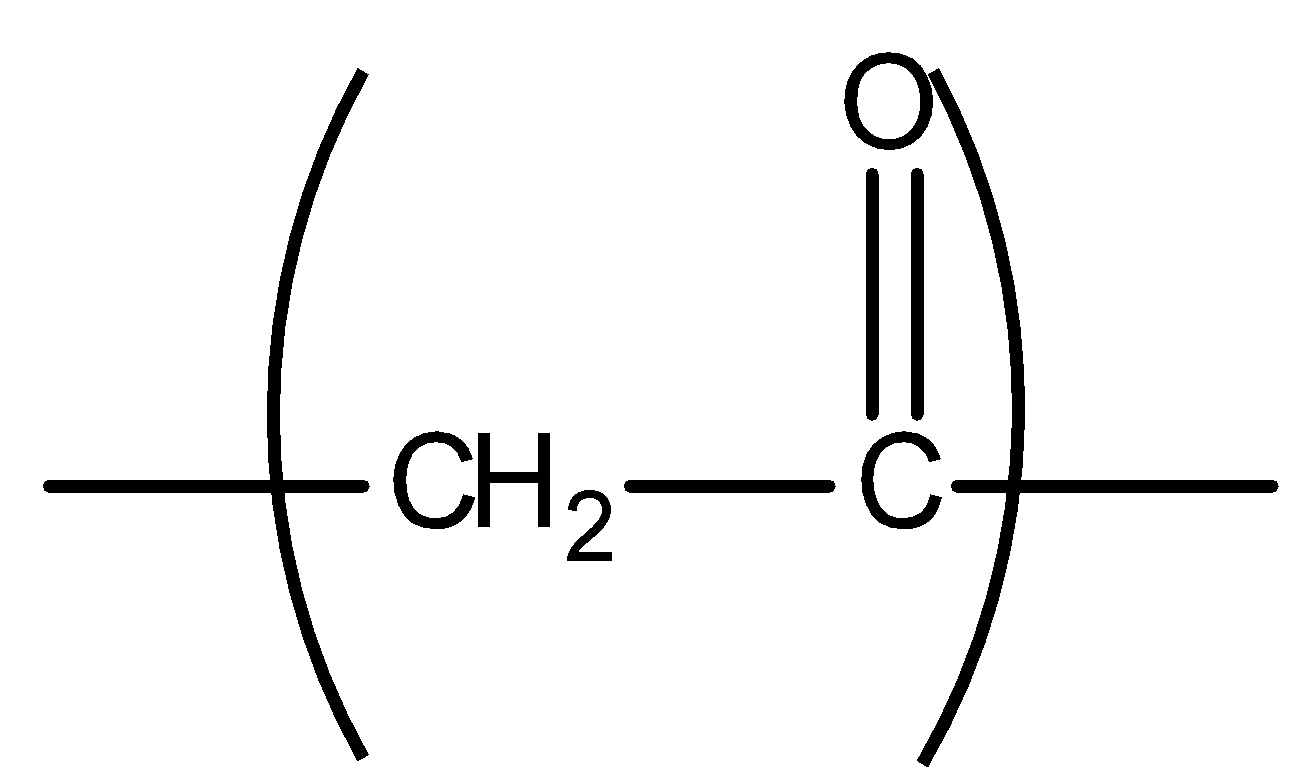
All the compounds having methyl ketone groups give iodoform tests. Ketogenic groups are as follows:
Now, compound I i.e.,
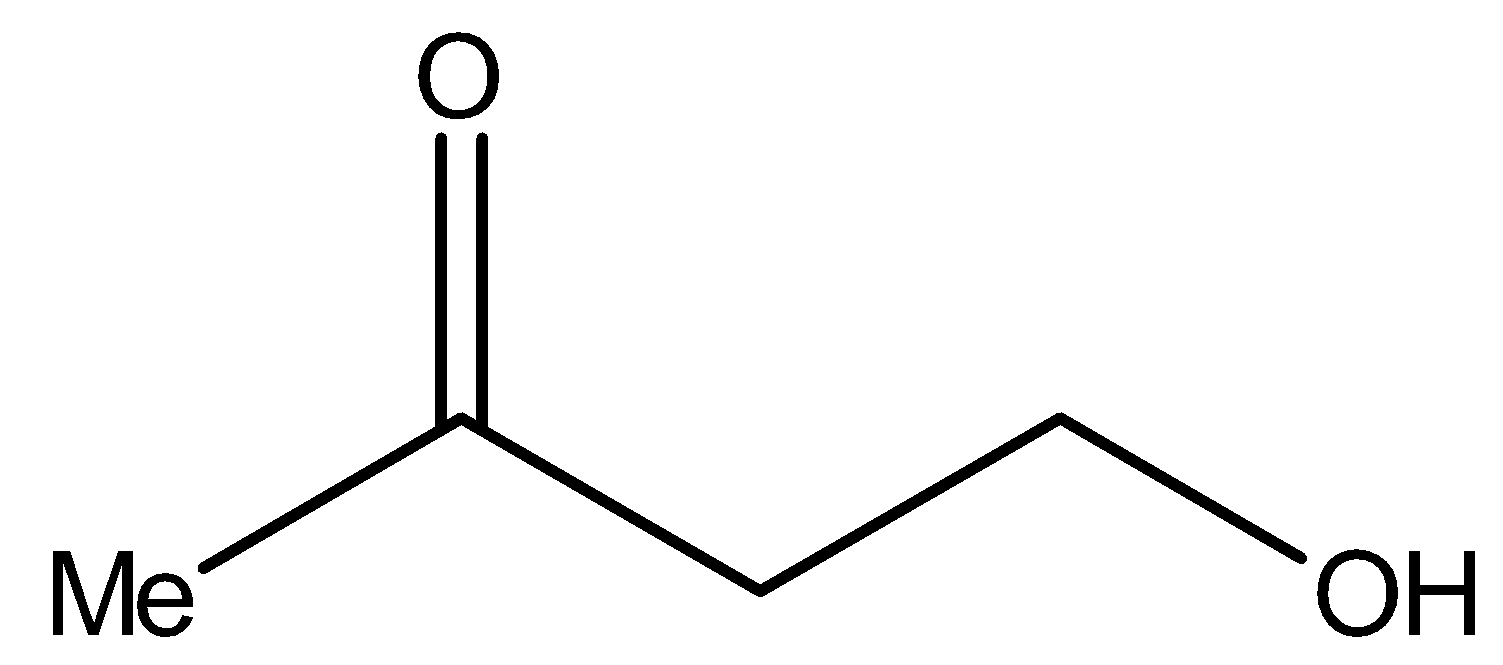
It contains methyl ketonic group (−CH2CO−). Therefore, it will give iodoform test.
Similarly, compound II i.e.,
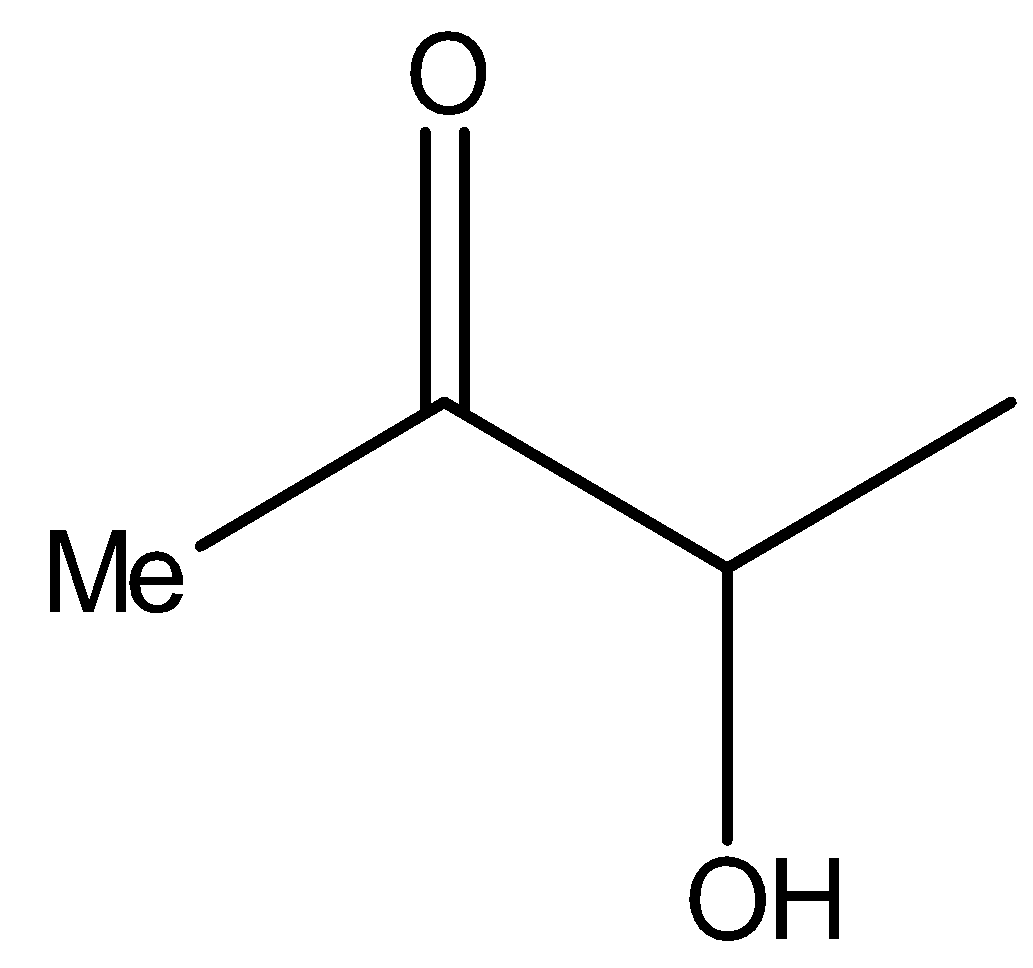
It also contains methyl ketone groups. Hence, compound II will also give iodoform test.
We know that alcohols give turbidity with Lucas reagent. Since compound I contains alcoholic groups (-OH), it will give turbidity with Lucas reagent.
All aromatic and aliphatic aldehydes reduce Tollens’ reagent and give a silver mirror. But ketones of special class i.e., α- hydroxy ketones reduce Tollens’ reagent. General representation for α- hydroxy ketones is:
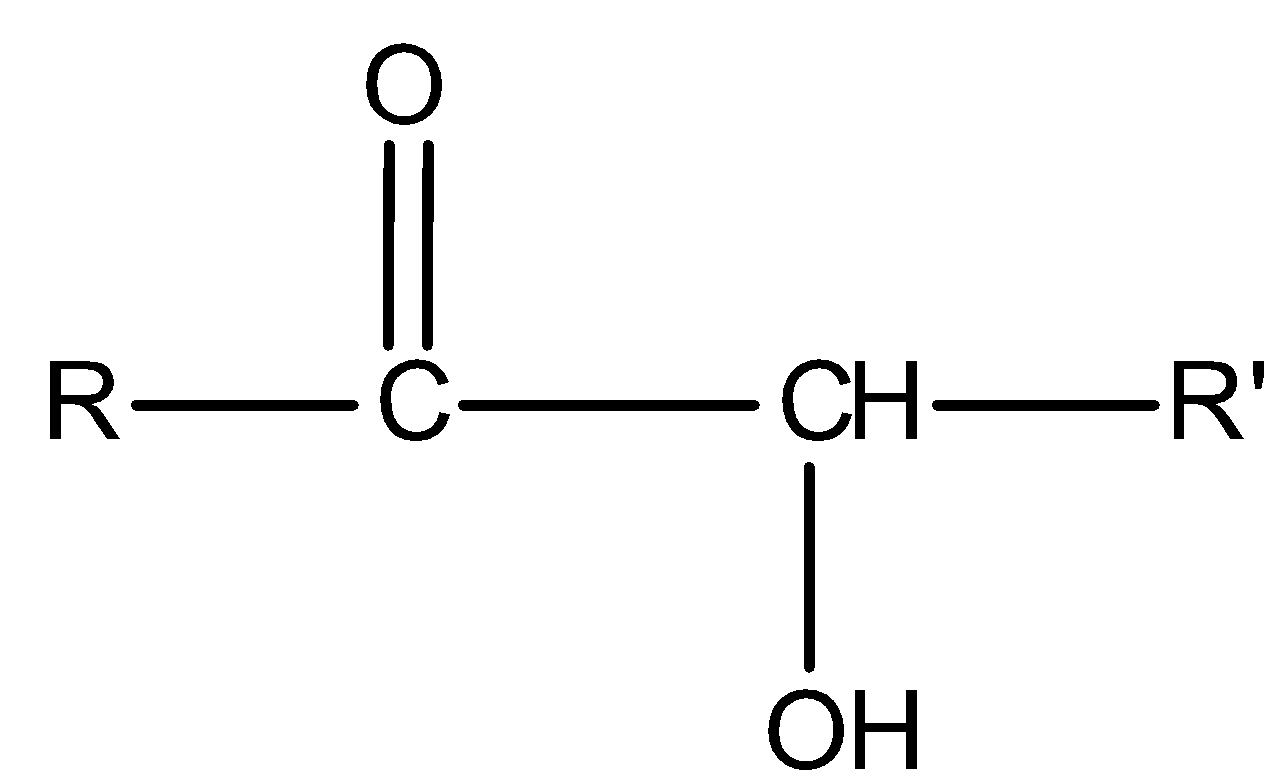
Now see the compound II,
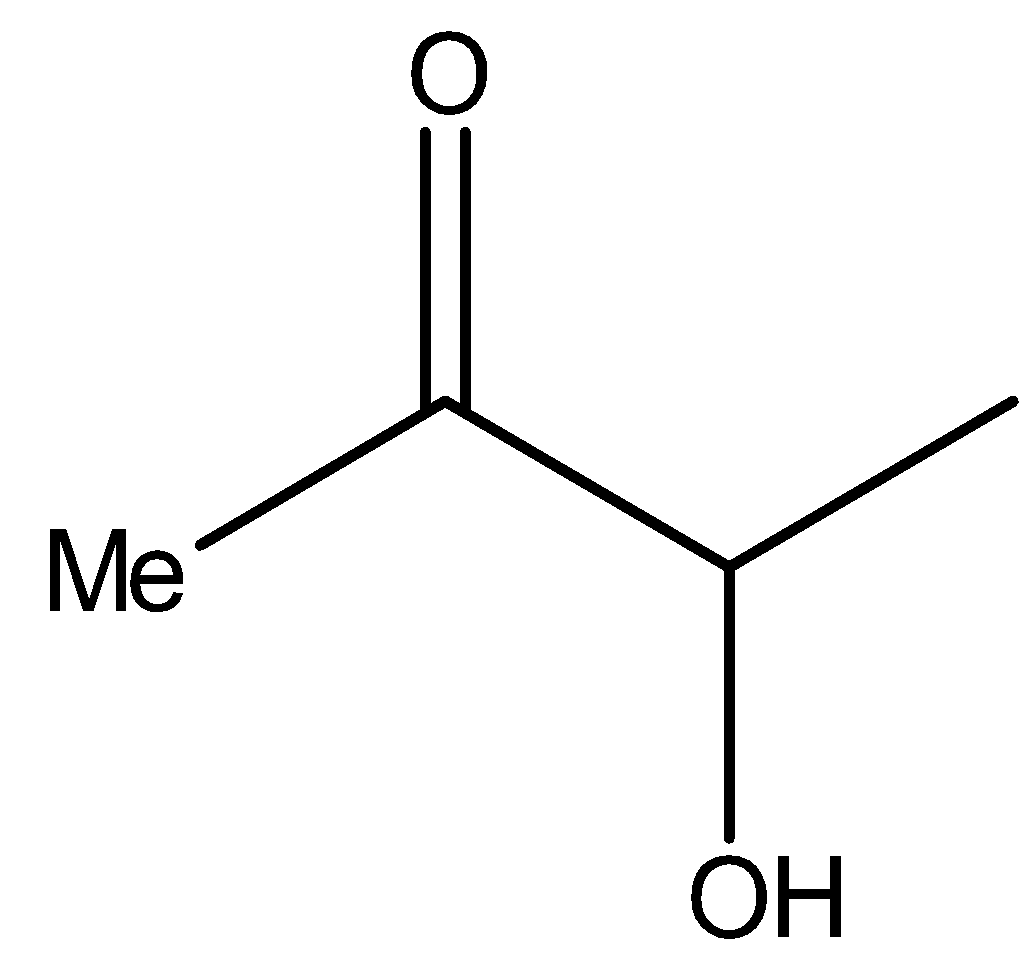
It is the same as the structure above, i.e., it is a α- hydroxy ketone. Therefore, compound II will reduce Tollens’ reagent.
Thus, statement A is correct.
B) Compound III gives a silver mirror with [Ag(NH3)2]⊕ and does not react with NaOBr.
Compound III is:
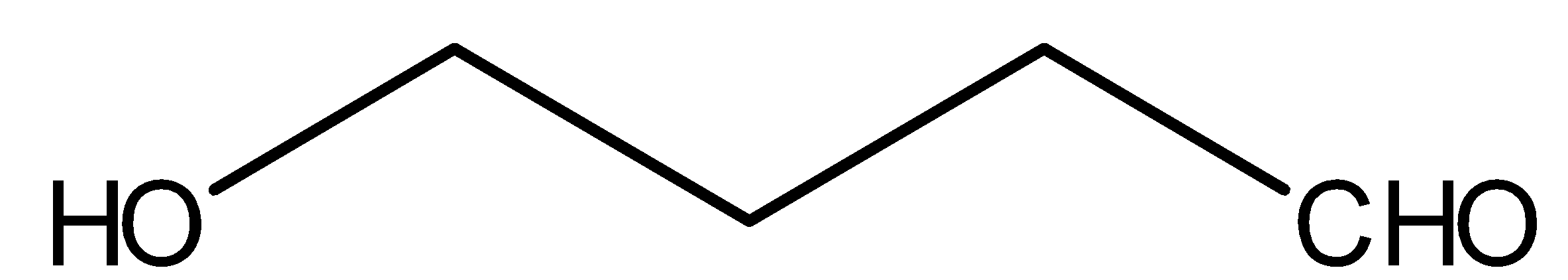
This above compound is an aldehyde, therefore it will reduce Tollene’s reagent and will give silver mirror. Tollen’s reagent is [Ag(NH3)2]⊕. NaOBr is nothing but combination of NaOH and Br2. Methyl ketones gives react with NaOBr. But compound III does not have methyl ketonic group therefore, it will not react with NaOBr.
Thus, statement B is correct.
C) Compound IV on acid hydrolysis gives C2H5COOH and MeOH.
Acidic hydrolysis (H+/H2O) of compound IV:
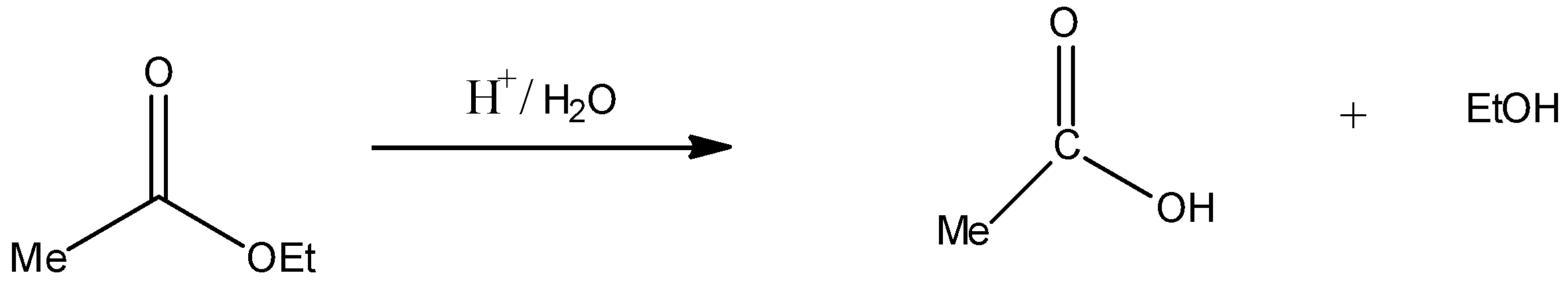
Thus, compound IV on acid hydrolysis gives MeCOOH and EtOH. Hence, statement C is incorrect.
D) Compound V on heating is decarboxylated to propane.
Compound V is:
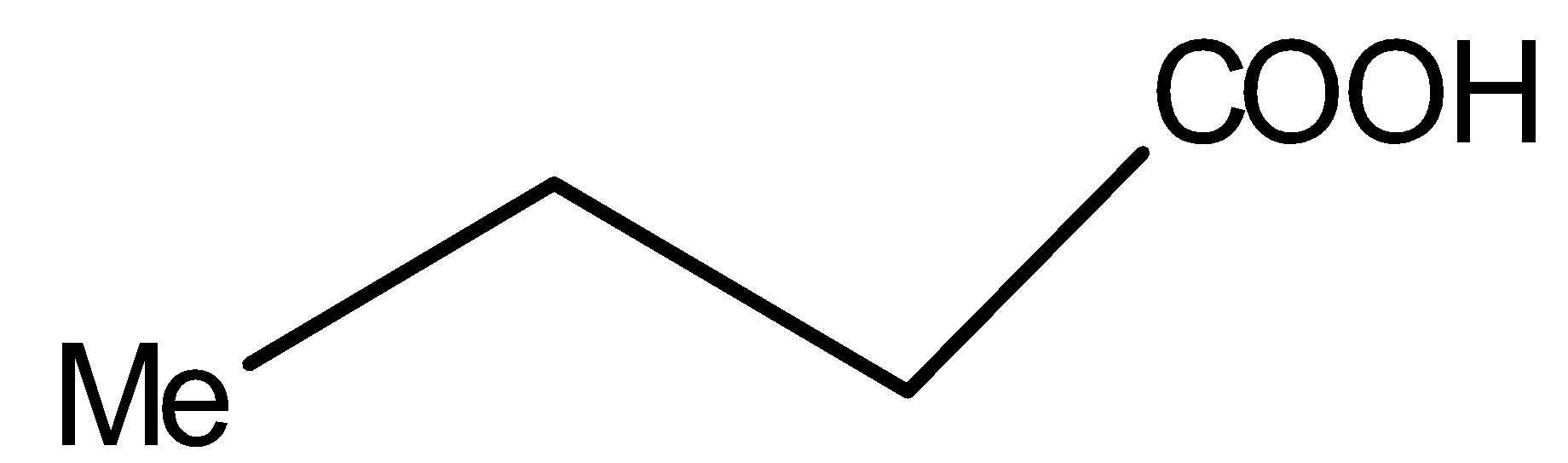
You must know that only β- keto acids under decarboxylation. General representation of β- keto acids is:
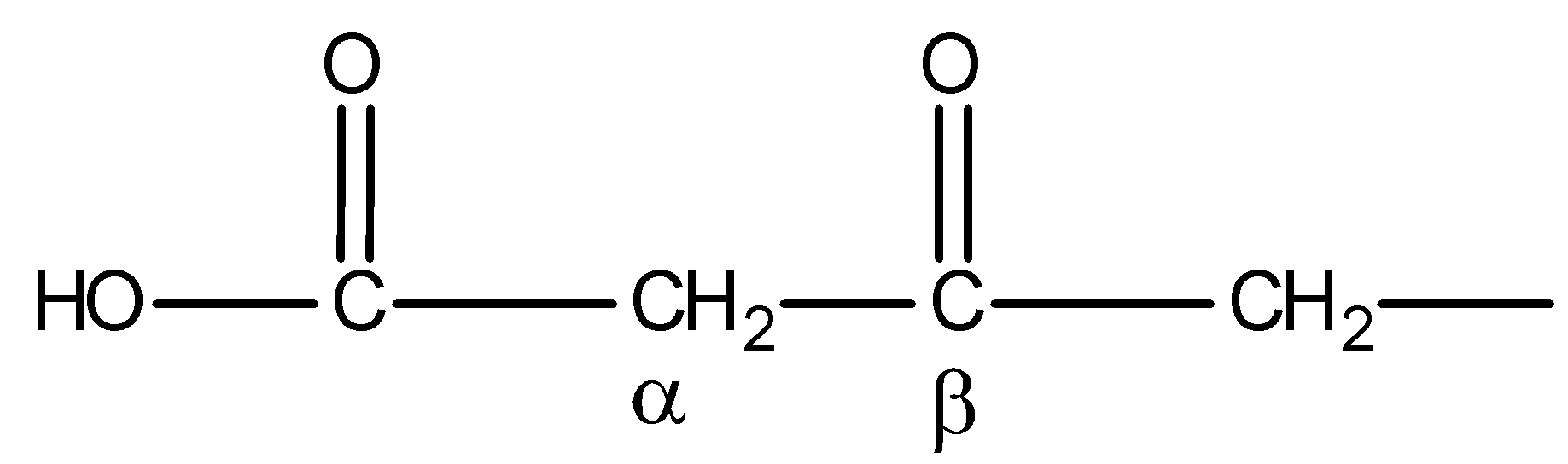
But compound IV is not β- keto acid. Thus, on heating, it will not undergo decarboxylation. Hence, statement D is incorrect.
Therefore, among all the given statements, statement A and B are correct. Thus, option A and B are the answers.
Note: Lucas reagent is a solution of anhydrous zinc chloride (ZnCl2) in conc. hydrochloric acid. Lucas reagent is used to distinguish the primary, secondary and tertiary alcohols. All alcohols show turbidity with Lucas reagent but they differ in the time taken for showing the turbidity.
