Question
Question: Compound A of molecular formula \({{C}_{3}}{{H}_{6}}O\) does not reduce Tollens reagent and Fehling’...
Compound A of molecular formula C3H6O does not reduce Tollens reagent and Fehling’s solution. Compound A undergoes Clemmensen reduction to give compound B of the molecular formula C3H8 . Compound A in the presence of conc. H2SO4 condenses giving aromatic compound C of the molecular formula C9H12 . Identify B and C. Explain the reactions.
Solution
Clemmensen reduction is a chemical reaction for the reduction of ketones or aldehydes to alkanes using zinc amalgam and concentrated HCl. Fehling’s solution besides Tollen’s reagent is used to differentiate between reducing and non reducing sugars.
Complete answer:
- Tollen’s reagent is a chemical reagent used to identify the presence of aldehyde, aromatic aldehyde, and alpha-hydroxy ketone functional groups.
- Fehling is a deep blue alkaline solution which is used to determine the presence of aldehyde or ketone groups.
- Clemenson reductions are effective at reducing aryl-alkyl ketones.
-Since the compound, A with molecular formula C3H6O is not reduced by Tollen’s reagent and Fehling’s solution and is undergoing Clemmensen reduction, so it must be ketone (acetone).
-Acetone is further reacted with conc. H2SO4 with and distilled water gives Mesitylene which is the compound C. It has the molecular formula as C9H12 and is an aromatic compound.
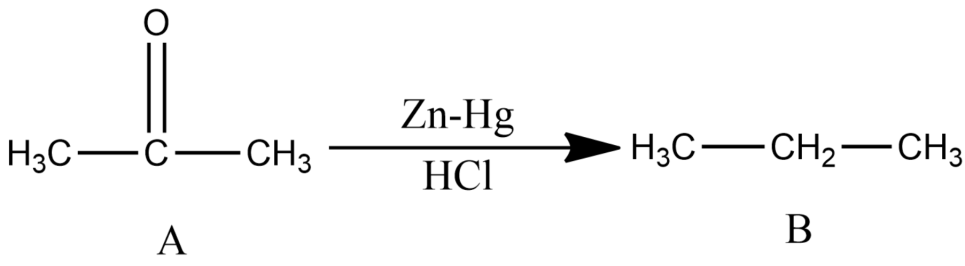
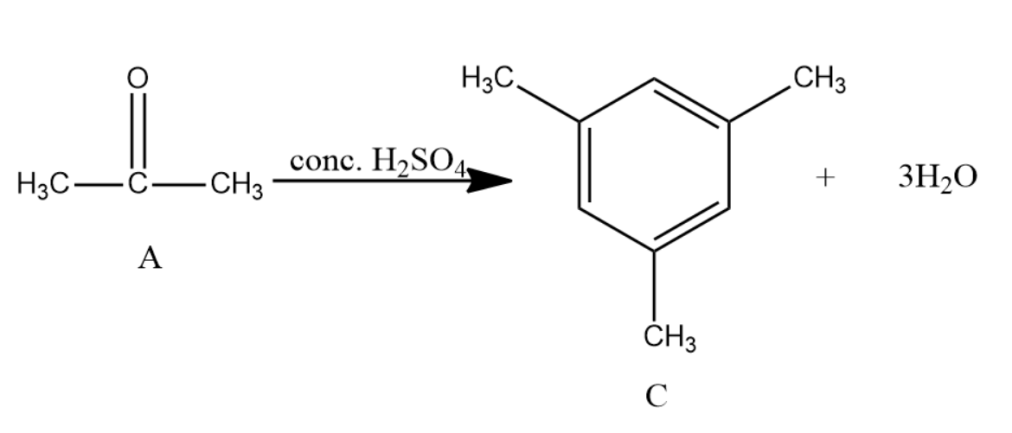
Note: You may get confused about compound A thinking it as aldehyde or ketone because the Clemmensen reaction is the reduction reaction for both aldehyde and ketone. Since compound A is not giving any reaction with Tollen’s reagent and Fehling’s solution, this cancels out the chances of compound A being an aldehyde.
