Question
Question: Compound A is used as a solvent in cough syrup. A. write the equation for the combustion of A. B...
Compound A is used as a solvent in cough syrup.
A. write the equation for the combustion of A.
B. when A is heated with concentrated sulphuric acid, water is removed and gas B is formed.
identify A and B. write the equation for the reaction.
C. write the electron dot structure of B
D. name the type of reaction B undergoes with hydrogen.
Solution
In the given question the following compound is used in making cough syrup and we have to name it and also have to give the combustion reaction of the compound followed by the equation and the electron dot structure.
Complete Step by step answer: (A)Compound A which serves as a solvent in a cough syrup is an ethanol.
Compound A = C2H5OH
So, the reaction arise for combustion of ethanol will be
C2H5OH + 3O2→2CO2+ 3H2O
So, when we heat the ethanol in presence of concentrated sulfuric acid the removal of water molecule that is dehydration occurs and unsaturated hydrocarbon that we call ethene gas is produced.
(B)So, the reaction occur will be
C2H5OH + H2SO4→CH2= CH2+H2O
So, the Compound B is Ethene = C2H4
(C)The electron dot structure of the Compound B that is Ethene is as follows-
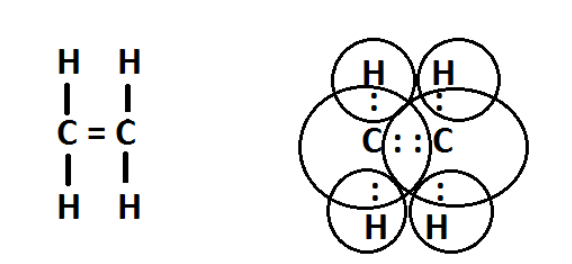
Figure-Electron dot structure of ethene
(D)When Ethene reacts with hydrogen gas it undergoes an additional reaction in which the hydrogen atom gets added to the Ethene molecule to give ethane. This type of reaction is termed a Hydrogenation.
The reaction which occurs is given by-
CH2= CH2+ H2→C2H6
Note: As Ethanol is used in cough syrup because it acts as the bronchodilator which is used to relax the muscles of bronchioles. Though the Ethanol is a fermented natural byproduct of a plant which also can be produced through the hydration of ethylene.
