Question
Question: Compound [A] is an aromatic amine which react with \(NaN{O_2} + HCl\) at \(273 - 278K\) and form com...
Compound [A] is an aromatic amine which react with NaNO2+HCl at 273−278K and form compound [B].Compound [B ] react with HBF4 and the obtain product on further heating, in the presence of NaNO2 and confirm product [C]. Compound [C] reduced in the presence of Sn/HCl to re-formed compound [A]. Write the general name of A, B, and C and write the equation of all reactions involved.
Solution
We have to remember that the anilines are the natural mixtures in the class of gathering coming in natural science which are likewise called as aminobenzene or phenyl amine. These mixtures are supposed to be harmful in nature and furthermore known to be one of the classes of fragrant amines. These are utilized in a wide assortment of mechanical and are known to have every one of the qualities of a sweet-smelling compound. The aniline compounds are said to have the recipe C6H5NH2 wherein the amino gathering should be appended to the Phenyl group.
Complete answer:
We also remember that the substance interaction utilized in changing over an essential fragrant amine into the related diazonium salt of the amine is generally alluded to as diazotization. This interaction is otherwise called 'diazotization'. The readiness of these diazonium salts includes the response of a sweet-smelling amine with nitrous acid within the sight of another acid.
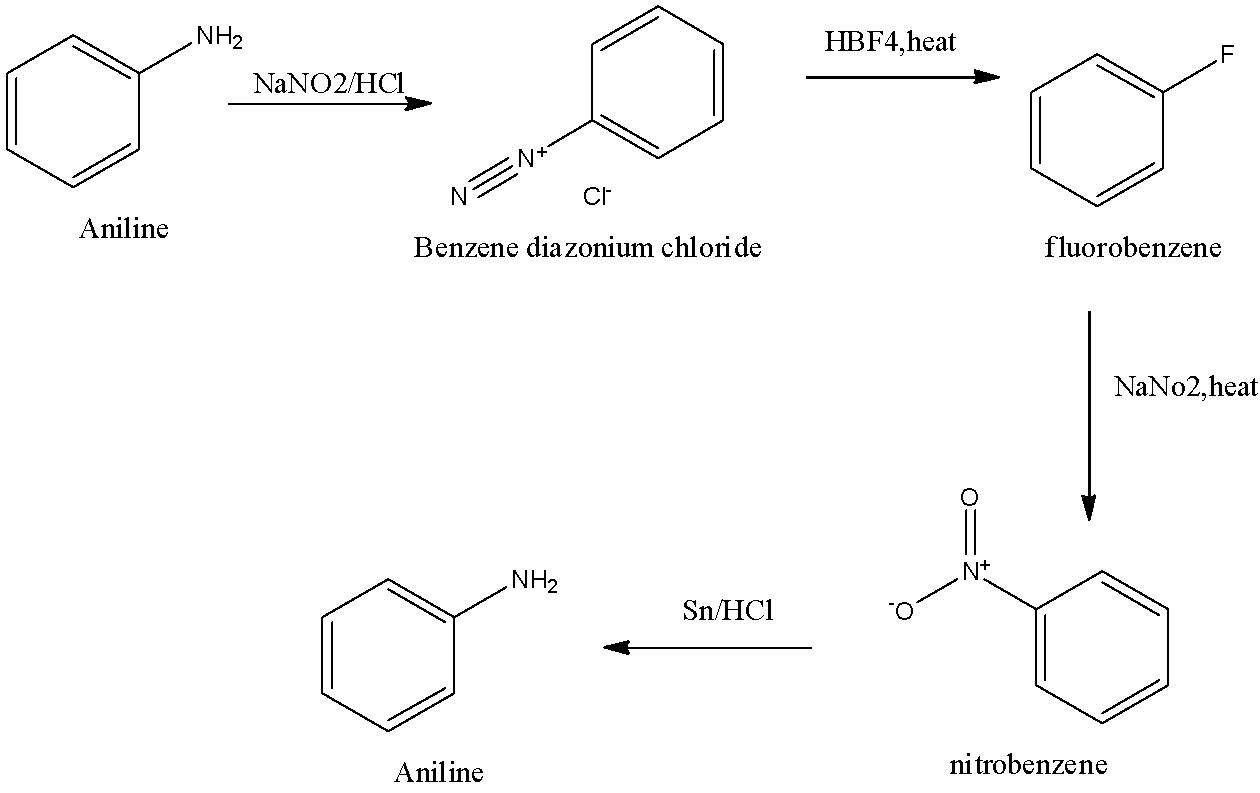
Additional information:
As we know that a couple of uses of diazonium compounds have been recorded underneath.
Diazonium compounds are helpful in the color and shade businesses. They were at first utilized in the creation of water-quick colored textures.
Diazonium Salts are discovered to be valuable in the Fischer Indole Synthesis measure since they can be diminished to hydrazine subordinates with the assistance of stannous chloride.
They are utilized as standard reagents while blending natural mixtures.
Diazonium salts can possibly have applications in the field of nanotechnology. They are helpful in the productive Functionalization of single divider nanotubes.
Note:
We also remember that the diazotization reaction system for the most part includes the utilization of nitrous acid and another acid in the treatment of aromatic amines to yield the diazonium salt. The German modern physicist Peter Griess was the primary individual to report such a response in 1858. He proceeded to find a lot more responses including diazonium salts.
