Question
Question: Compound A (\({C_9}{H_{10}}O\)) shows positive iodoform test. Oxidation of A with \(KMn{O_4}/KOH\) g...
Compound A (C9H10O) shows positive iodoform test. Oxidation of A with KMnO4/KOH gives acid B (C8H6O4). Anhydride of B is used for the preparation of phenolphthalein. Compound A is:
phenolphthalein. Compound A is:
(A) 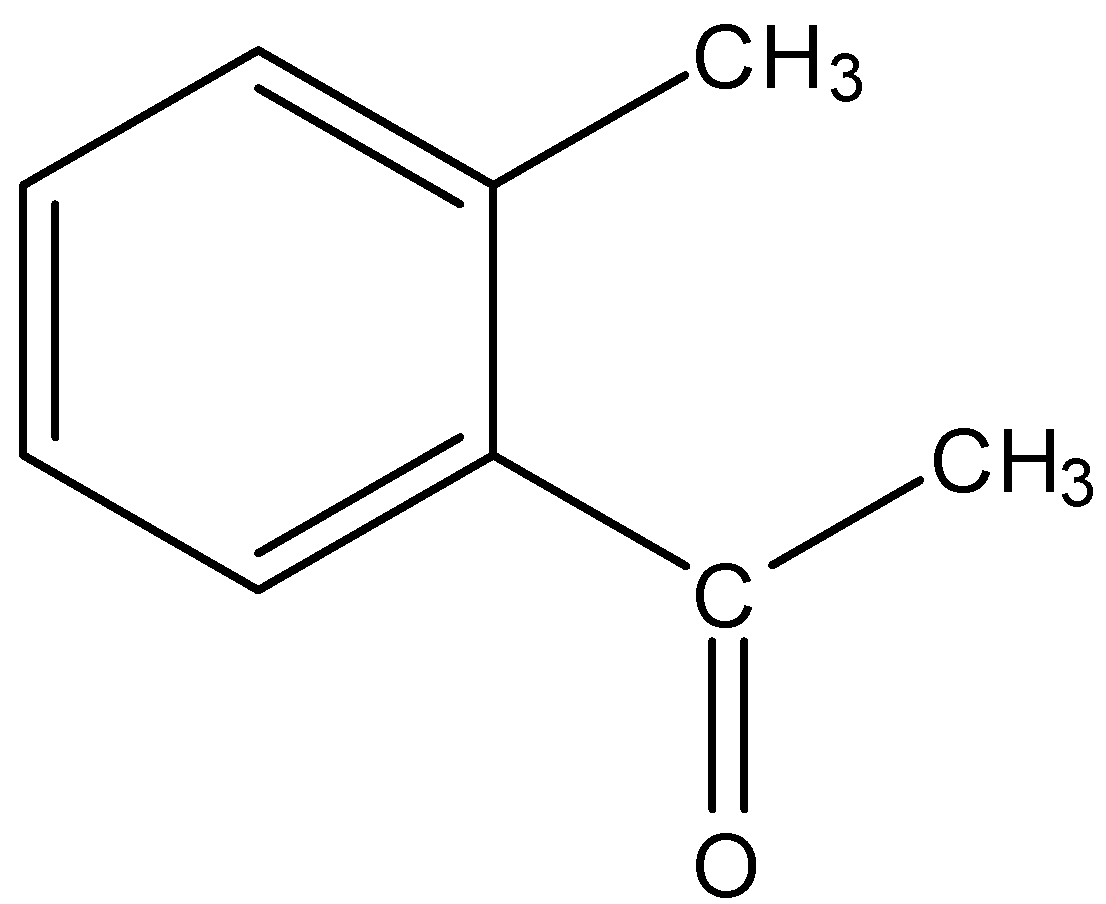
(B) 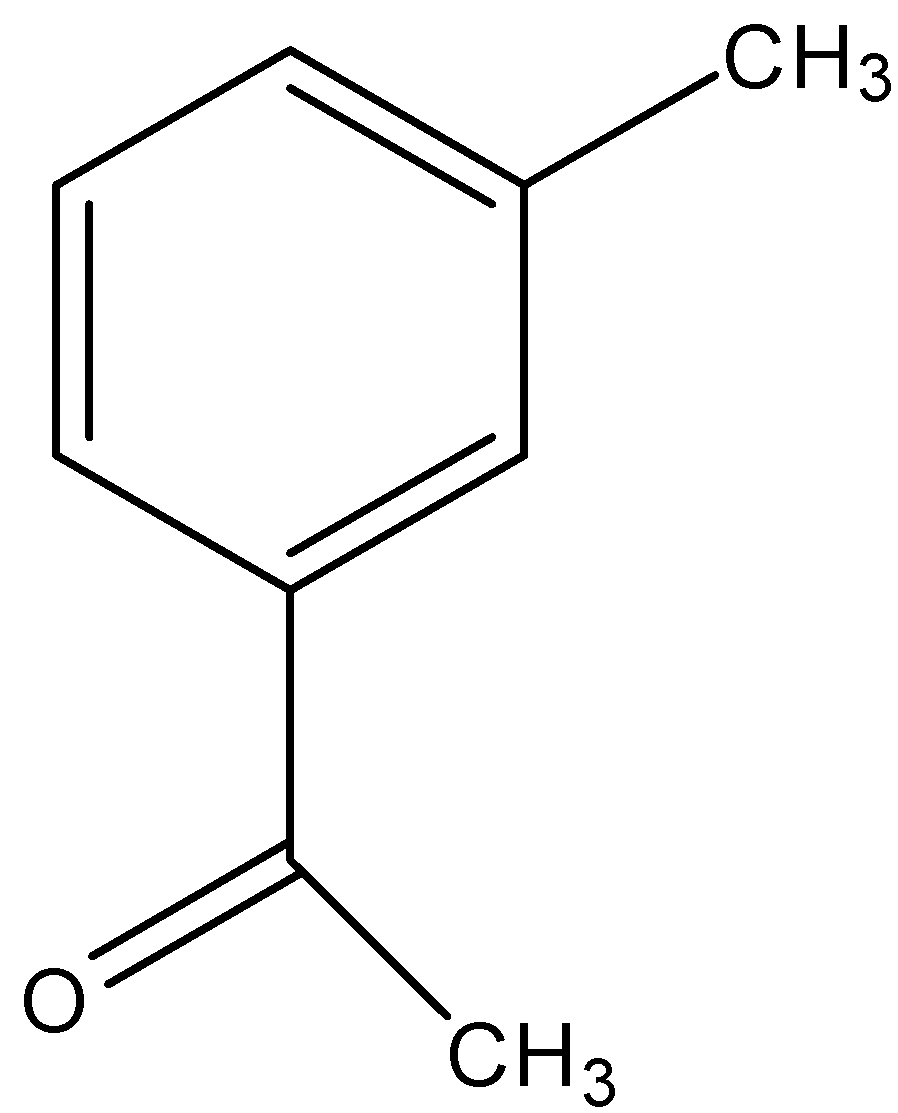
(C) 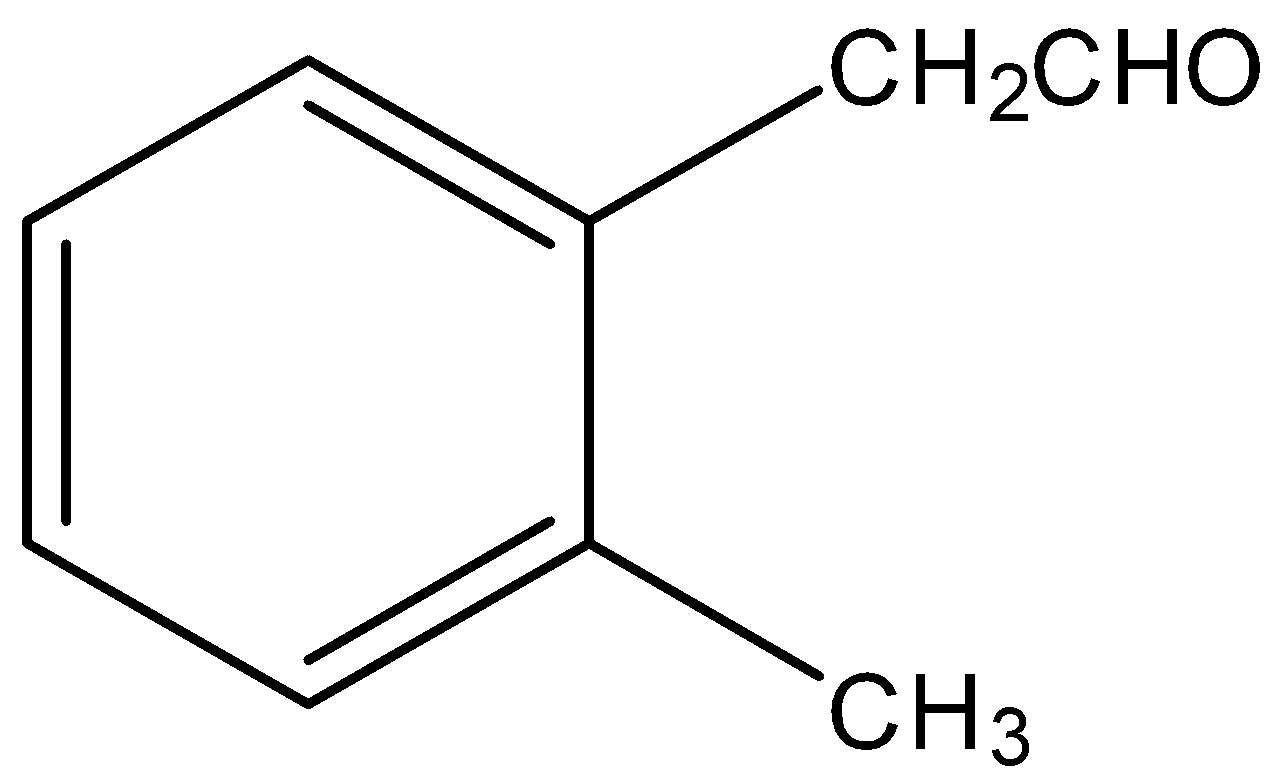
(D) 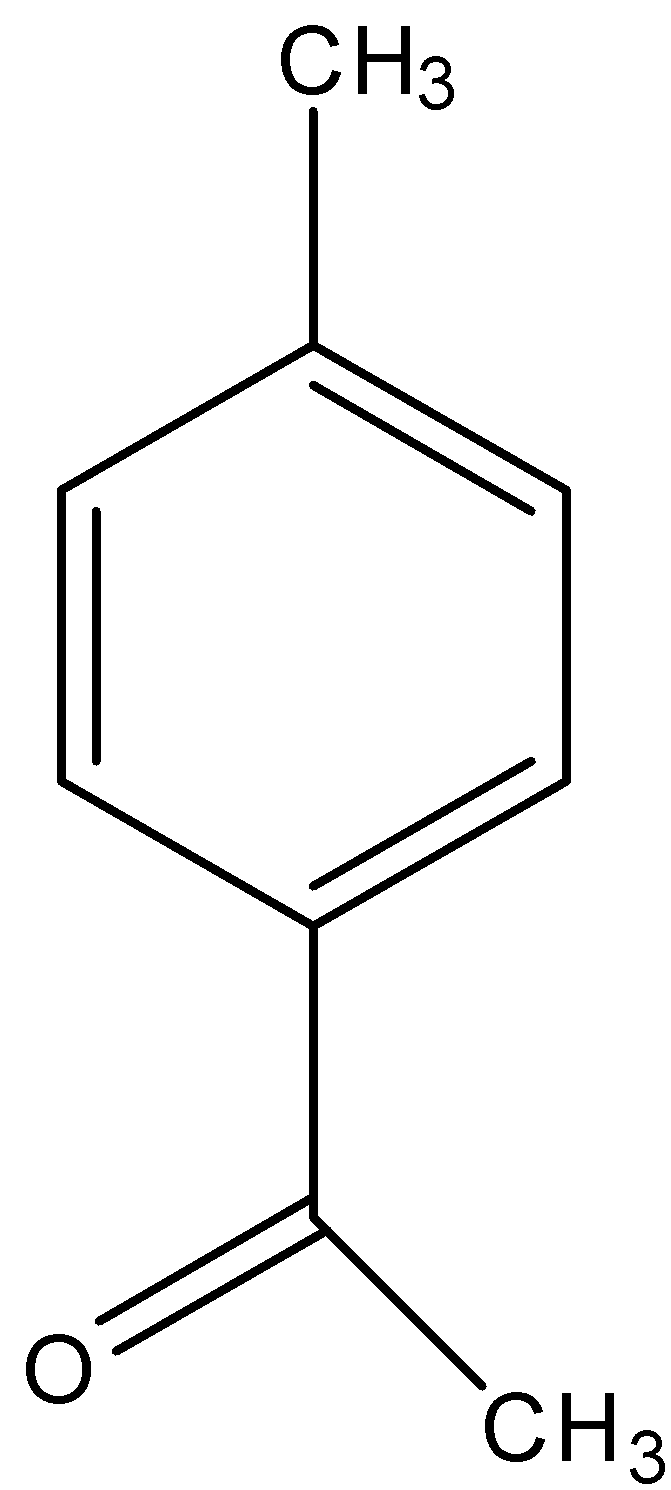
Solution
. The compounds which have methyl ketone in their structure, can give positive iodoform tests. The acid produced here upon the reaction of compound A with alkaline potassium permanganate solution is a dicarboxylic acid.
Complete step by step answer:
It is given to us that the compound A gives a positive iodoform test.
- We know that only the methyl ketones give positive iodoform tests. Methyl ketones have a −COCH3 group. The iodoform test can be given as under.
R−COCH3I2NaOHR−COOH+CHI3
Thus, we can say that compounds given in option A, B and D can give positive iodoform tests. Compounds in option C cannot give positive iodoform test because it does not have −COCH3 group.
- It is given that oxidation of A with potassium permanganate in an alkaline medium gives an acid. Further it is given that the acid is used in the preparation of phenolphthalein. We will see how phenolphthalein is produced.
- Now, we will find out which of the compounds can give an acid from which we can obtain phthalic anhydride.
- KMnO4 in alkaline medium (Here KOH) can oxidize the alkyl group on the benzene ring to give a carboxylic acid functional group. So, compound given in option (A) will give a reaction as given below.
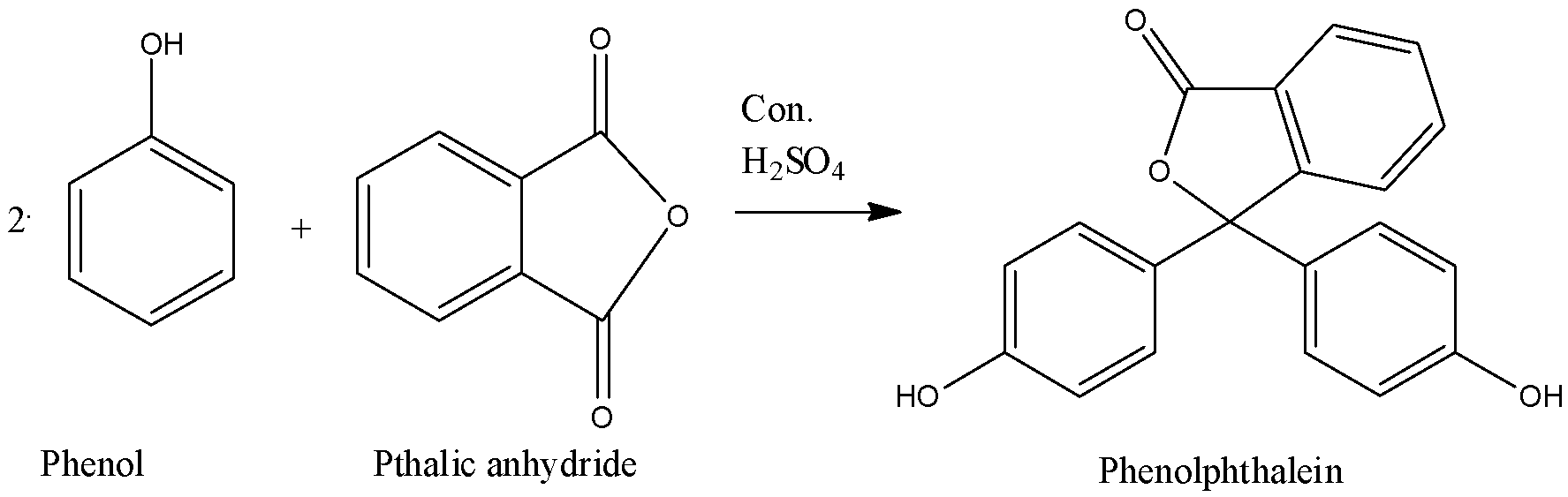
Thus, we can find that the anhydride is phthalic anhydride which is used to prepare phenolphthalein. We can see that phenol reacts with phthalic anhydride in presence of concentrated sulphuric acid to give phenolphthalein.
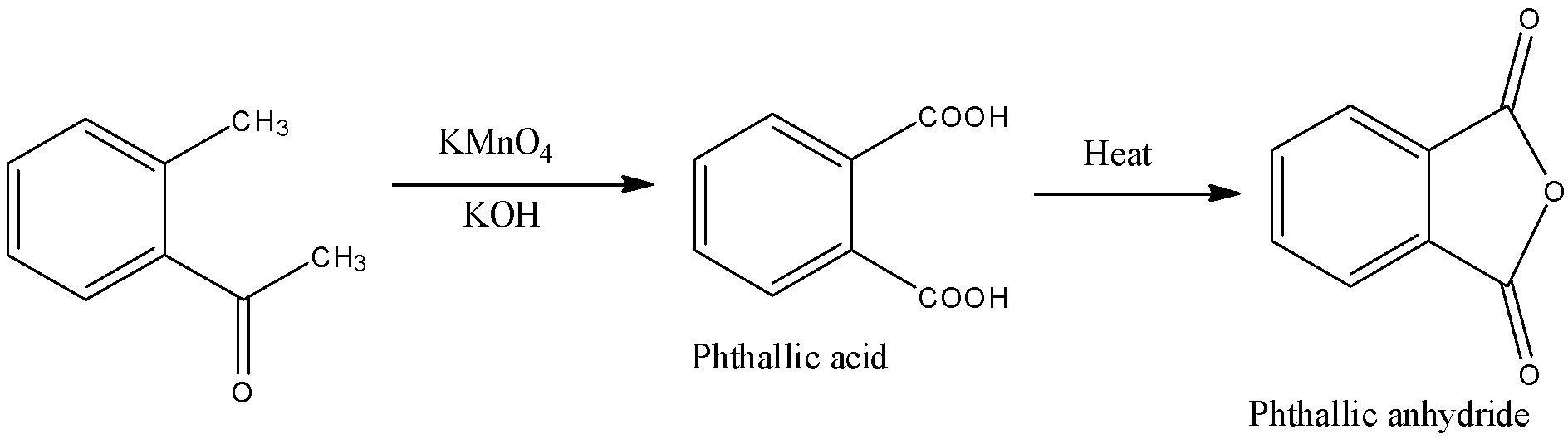
Here, we can see that a dicarboxylic acid is produced which has a molecular formula of C8H6O4. When it is heated, phthalic anhydride is produced.
So, the correct answer is “Option A”.
Note: Remember that even the acyl group (−COCH3) attached to benzene ring gets oxidized to carboxylic acid group (−COOH) upon its reaction with alkaline solution of potassium permanganate.
