Question
Question: Compound (A), \({{C}_{8}}{{H}_{9}}Br\), gives a pale yellow color precipitate when warmed with alcoh...
Compound (A), C8H9Br, gives a pale yellow color precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
a.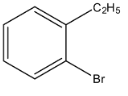
b.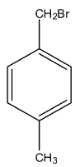
c. 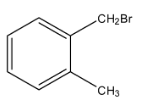
d.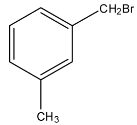
Solution
In the question, the compound (A) can form a yellow precipitate with silver nitrate, this is due to the fact that there is the formation of silver bromide, this can be formed only when the bromine atom from the compound can be easily released, if the bromine atom is attached with the benzene ring then, it will not easily release.
Complete answer: In the question, the compound (A) can form a yellow precipitate with silver nitrate, this is due to the fact that there is the formation of silver bromide, this can be formed only when the bromine atom from the compound can be easily released, if the bromine atom is attached with the benzene ring then, it will not easily release.
So, from the given options, (a) is not correct.
The second part of the question says that oxidation forms acid and it can easily form the anhydride. An anhydride can be formed from the compound only if both the acid groups are very near. Therefore, from the given options, only option (c), can form the anhydride.
The reactions are given below:
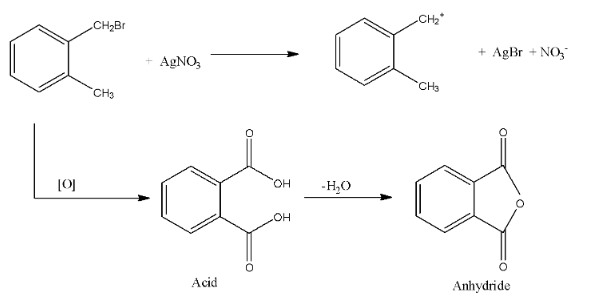
So, from the reaction, we can see that a correct answer is an option (c).
Note: If the acids on the benzene ring are present on the meta and para positions then, the anhydride cannot be formed, and anhydride is formed when one molecule of water is removed from the acid.
