Question
Question: Complex, charge of \({\text{Co}}\) is: 
A) +2
B) +3
C) +1
D) +4
Solution
To solve this we must know that the charge on the Co is the oxidation number of Co. Co is cobalt which is the central metal atom in the given complex. Remember there are two Co atoms. Four ammine (NH3), two sulphate (SO4), one hydroxide (OH) and one chloride (Cl) ligands are attached to the central metal atom i.e. Co.
Complete solution:
We are given a complex as follows:
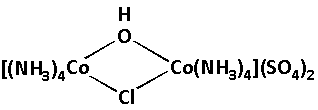
In the given complex, we have to calculate the charge of Co. The charge on the Co is the oxidation number of Co. Co is cobalt which is the central metal atom in the given complex. Also there are two cobalt atoms.
Different ligands are attached to the central cobalt atoms. Four ammine (NH3), two sulphate (SO4), one hydroxide (OH) and one chloride (Cl) ligands are attached to the central cobalt atoms.
Thus,
Charge on the complex=2(Co)+4(NH3)+2(SO4)+1(OH)+1(Cl)
Four ammine (NH3) ligands are attached. The amine ligands are neutral in nature. Thus, the charge on amine ligands is zero.
Two sulphate (SO4) ligands are attached. The sulphate ligands are negative in nature. Each sulphate ligand has a charge of −2.
One hydroxide (OH) ligand is attached. The hydroxide ligands are negative in nature. Each hydroxide ligand has a charge of −1.
One chloride (Cl) ligand is attached. The chloride ligands are negative in nature. Each chloride ligand has a charge of −1.
As there is no charge mentioned on the overall complex we consider that the total charge on the complex is zero.
Let the charge on the central cobalt atom be x. Thus,
0=2(x)+4(0)+2(−2)+1(−1)+1(−1)
0=2(x)+0−4−1−1
2(x)=+4+1+1
2(x)=+6
x=+3
Thus, in the complex, charge of Co is +3.
Thus, the correct option is (B)
Note: To solve this you must remember that there are two cobalt atoms. If you consider only one cobalt atom then you will get that the charge of Co is +6. Also, remember the charges on each of the ligands attached to the central cobalt atom.
