Question
Question: Complete the reaction: \({\text{N}}{{\text{H}}_4}{\text{CNO }}\xrightarrow{{{\text{heat}}}}{\text{...
Complete the reaction:
NH4CNO heat ?
Solution
In order to complete a reaction, we must first identify the basic ions or functional groups present in the compound and think of all the possible ways in which it can react to form suitable products. In all reactions which involve heating, mostly two products may be formed, where in the initial product on product on prolonged heating yields another new product.
Complete step by step solution:
The chemical name of NH4CNO is ammonium cyanate. It is a colourless inorganic compound with the structure given below.
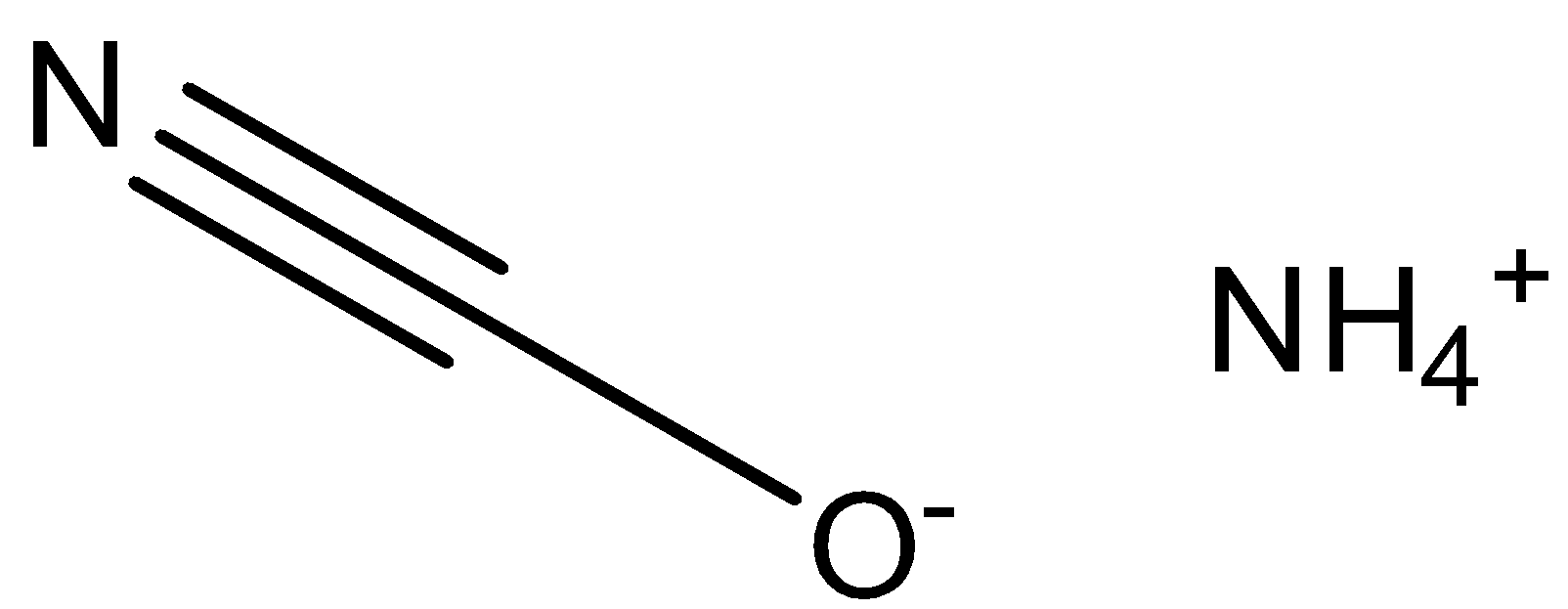
In order to draw the chemical structure of ammonium cyanate, we must first identify the individual constituents and their numbers present in the compound. Ammonium cyanate is a compound in which ammonium ion is attached with cyanate ion with a positive and negative charge respectively on the two ions present.
Ammonium cyanate on heating gives the compound known as urea. On prolonged heating of urea, a new product called biuret is formed.
The reactions can be depicted as follows:
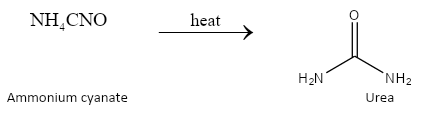
On prolonged heating, 2 molecules of urea combine together to produce biuret. We can depict the chemical reaction as follows:
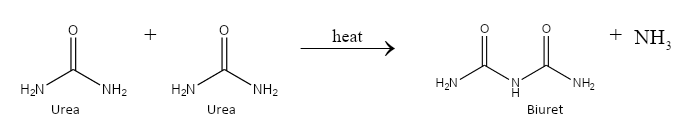
Hence, the correct answer is urea.
Additional Information:
Ammonium cyanate is an inorganic compound used as a precursor for the synthesis of urea through Wohler synthesis of urea, which is an organic compound.
Note:
Remember that ammonium cyanate is an inorganic compound and the product formed on heating ammonium cyanate is urea. The prolonged heating of urea leads to a product called biuret. Biuret test is a test which is performed to determine the presence of peptide bonds.
