Question
Question: Complete the reaction-
A.A is 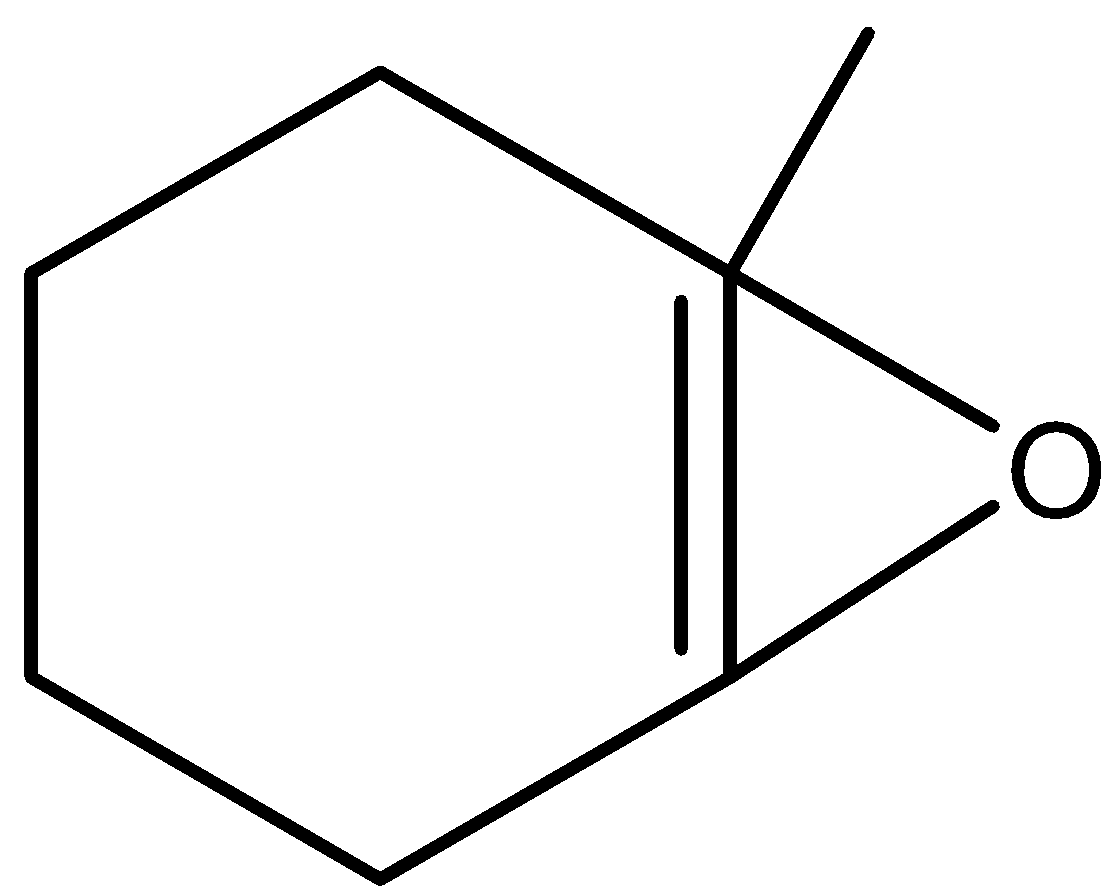
B.B is 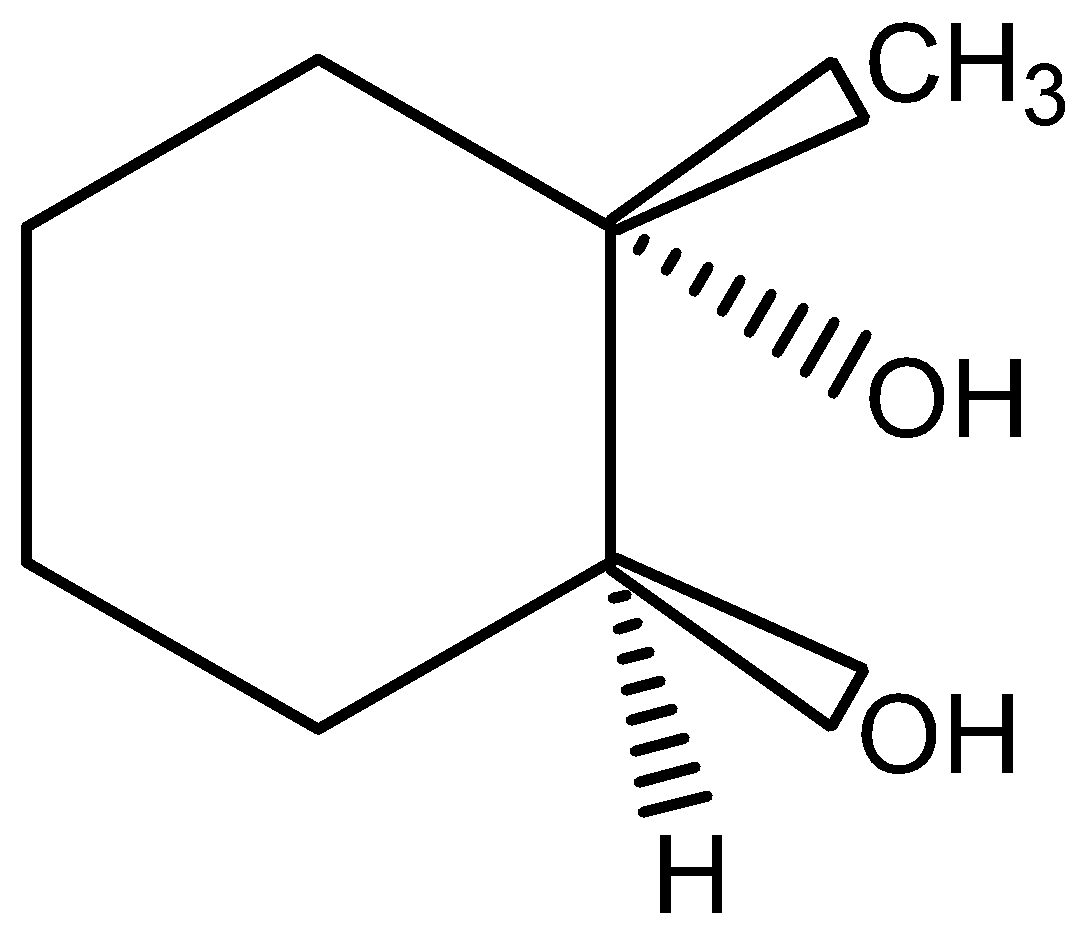
C.B is 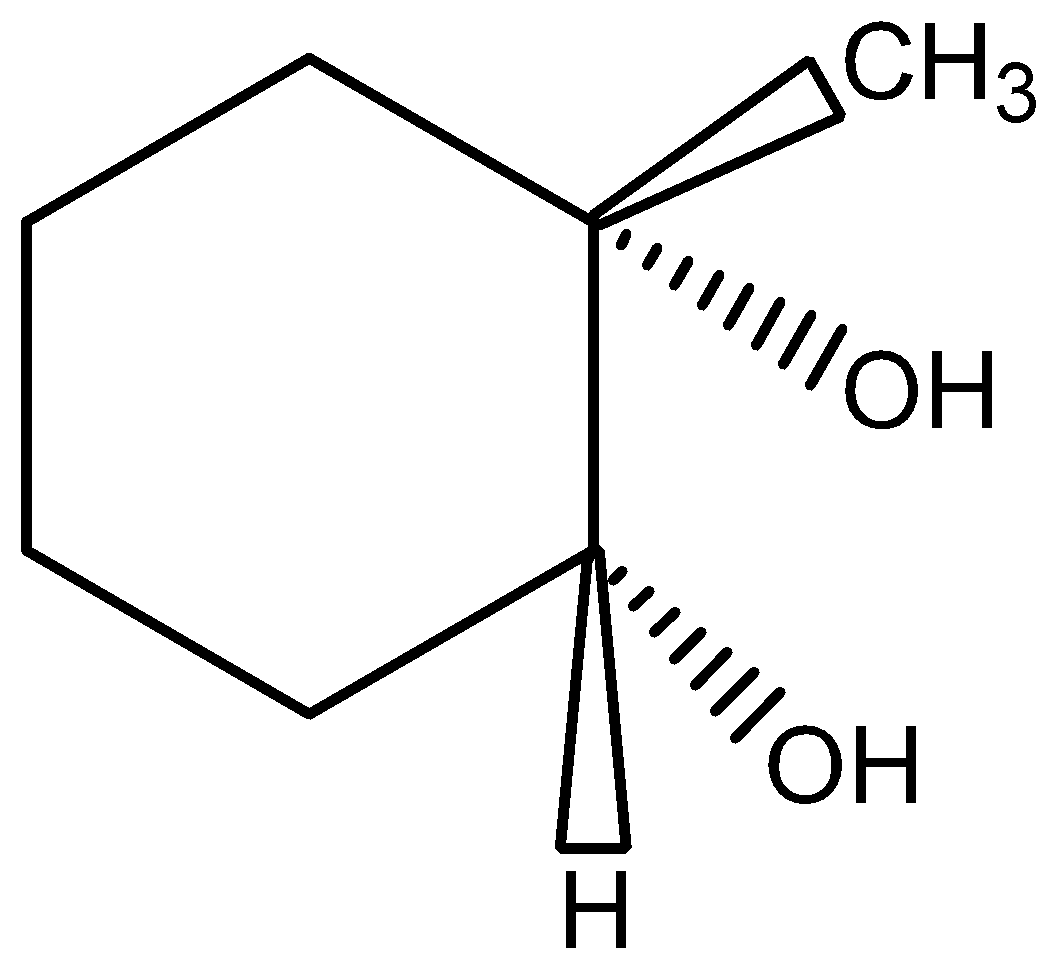
D.C is 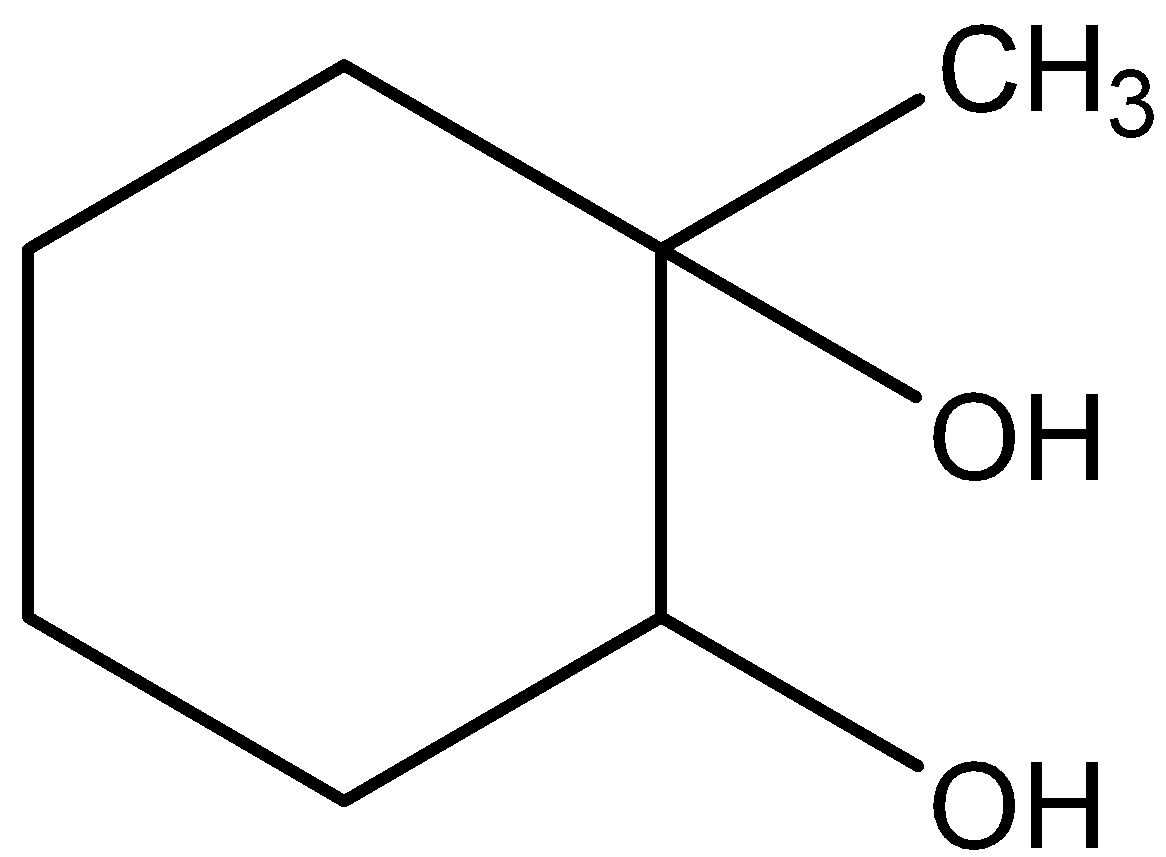
Solution
Here, the given reactant is 1-methylcyclohex-1-ene which is an organic compound having the molecular formula, C7H12. And 1-methylcyclohex-1-ene is an saturated hydrocarbon and it is mainly used as a solvent. Here, 1-methylcyclohex-1-ene is reacted with m-CPBA and there is a formation of two major products B and C.
Complete answer:
In this reaction, 1-methylcyclohex-1-ene is reacted with m-CPBA and this is considered as an oxidation reaction and the full conversion takes place. The full name of m-CPBA reagent is meta-Chloroperoxybenzoic acid which is mainly used as an oxidant during the synthesis of organic compounds.
Therefore, by the reaction of 1-methylcyclohex-1-ene with m-CPBA there is a formation of 1-methyl-7-oxabicycloheptane. And it undergoes hydrolysis and there is a formation of (2R)-1,2-dimethylcyclohexan-1-ol and (1S,2S)-1-methylcyclohexane-1,2-diol as major products. Let’s see the reaction,
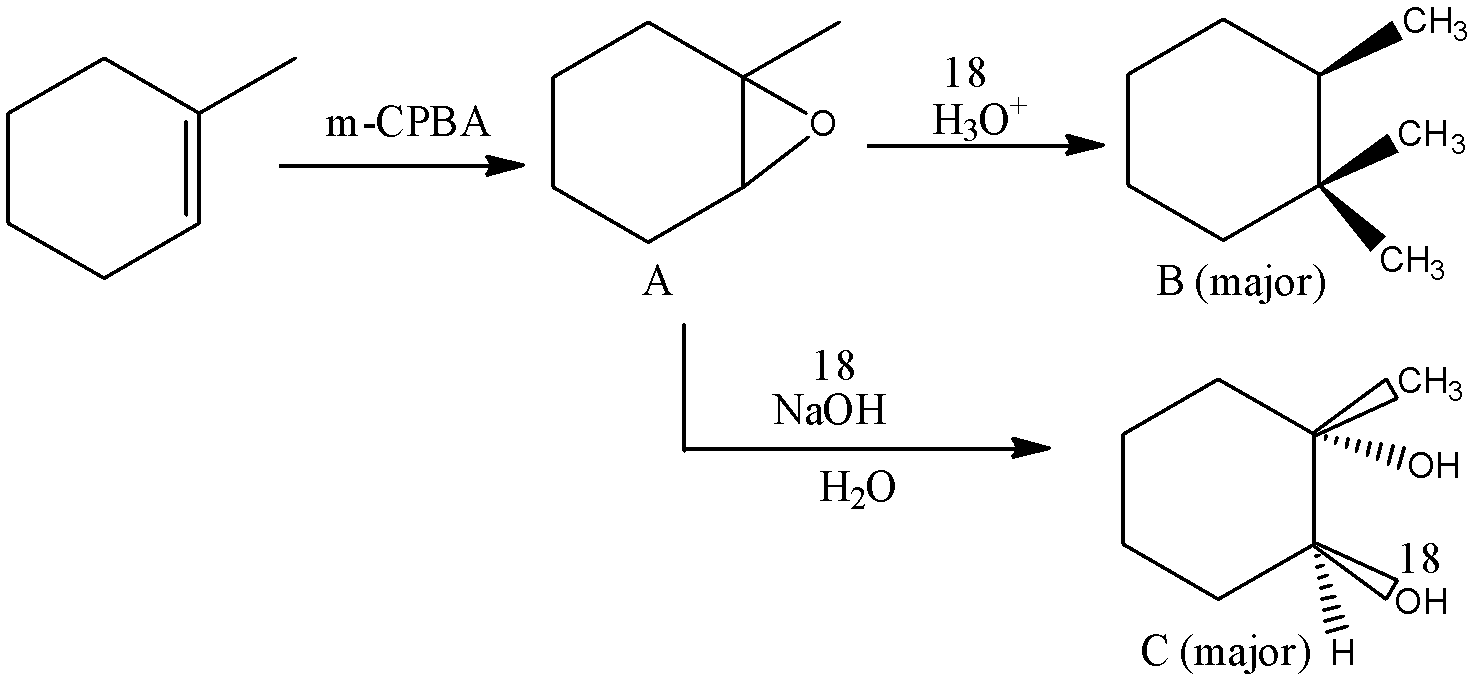
Hence, option (A) is correct.
This is not the correct structure of compound B. Hence, the option (B) is incorrect.
The compound B contains the chiral carbon in second position. Hence, option (C) is incorrect.
The compound C contains more than one chiral center. Therefore, it is not a correct structure of compound C.
Hence, the option (D) is correct.
Note:
Here, the cyclohexene is reacted in the presence of m-CPBA and there is a formation of two major products. And that both products have more one chiral centers. And this reaction is considered an oxidation reaction. Hence, the cyclohexane compound is converted into cyclohexanol. The m-CPBA is generally used as an oxidizing agent for organic synthesis.
