Question
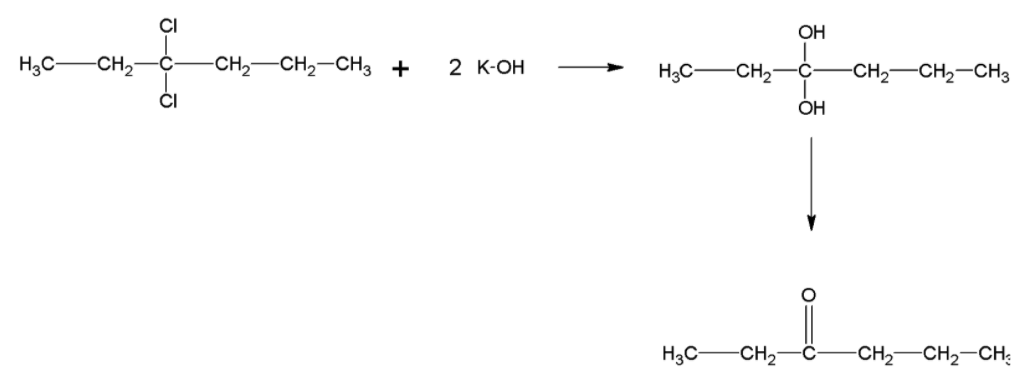
Question: Complete the reaction: 
Solution
To answer this question, you must recall the reactions taking place on use of different reagents. KOH is an ionic compound and in a polar medium like aqueous solution, it breaks up into its constituent ions forming a hydroxide ion.
Complete answer:
When KOH dissociates it forms hydroxide ion. OH− ion is both a strong base as well as a strong nucleophile. But in an aqueous solution, the hydroxide ions are highly hydrated. This reduces the basic character of OH− ions and thus it fails to abstract a hydrogen from the β- carbon of the alkyl chloride to form a double bond. So, it instead acts as a nucleophile which results in a nucleophilic substitution reaction. Both the chlorine molecules are replaced by a hydroxyl group each and a geminal diol is formed.
Oxygen atoms are large in size and have two lone pairs of electrons. Hence, when two hydroxyl groups are attached to a single carbon atom, it is highly unstable due to high lone pair- lone pair repulsion between the oxygen atoms. As a result, geminal diols undergo loss of a water molecule due to the vicinity of two hydroxyl groups to each other and a ketone is formed.
The final product obtained in the reaction is hexan-3-one.
Note:
On the other hand, if we use an alcoholic solution of KOH, the attacking species formed is different. In alcoholic solution KOH forms an alkoxide ion, which is a strong base. The alkoxide ion extracts a proton from the β- carbon atom of the alkyl chloride while the chlorine atom leaves as chloride ion. Hence, if the solution used was alcoholic KOH, then the compound would have undergone dehydrohalogenation reaction twice forming an alkyne.
