Question
Question: complete the reaction 
Solution
The presence of di-ols in presence of Conc. H2SO4 undergoes pinacol pinacolone type of rearrangement and forms pinacolone as a product. Pinacolone products contain a ketone and a tertiary carbon atom.
Complete answer:
The Pinacol Pinacolone rearrangement mechanism of the given compound contains four steps.
Step 1: The reaction is going to be carried out in an acidic medium, and then one of the hydroxide groups of the pinacol is going to be protonated by the acid.
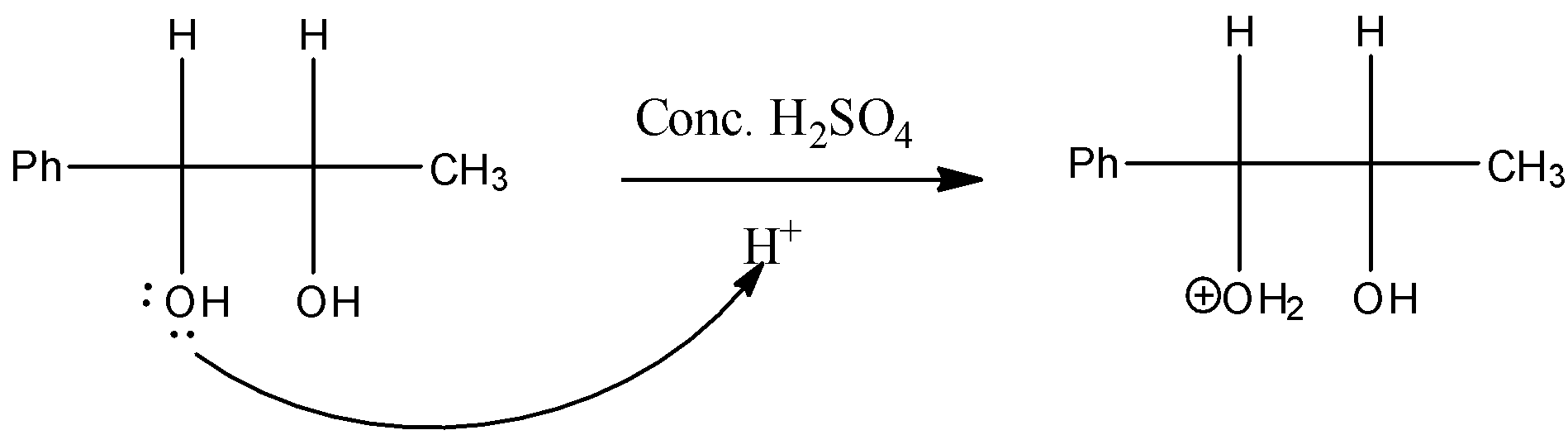
Step 2: Then the water molecule is removed from the compound, by creating a carbocation in the compound. Always tertiary carbocation is going to form because tertiary carbocation is more stable.
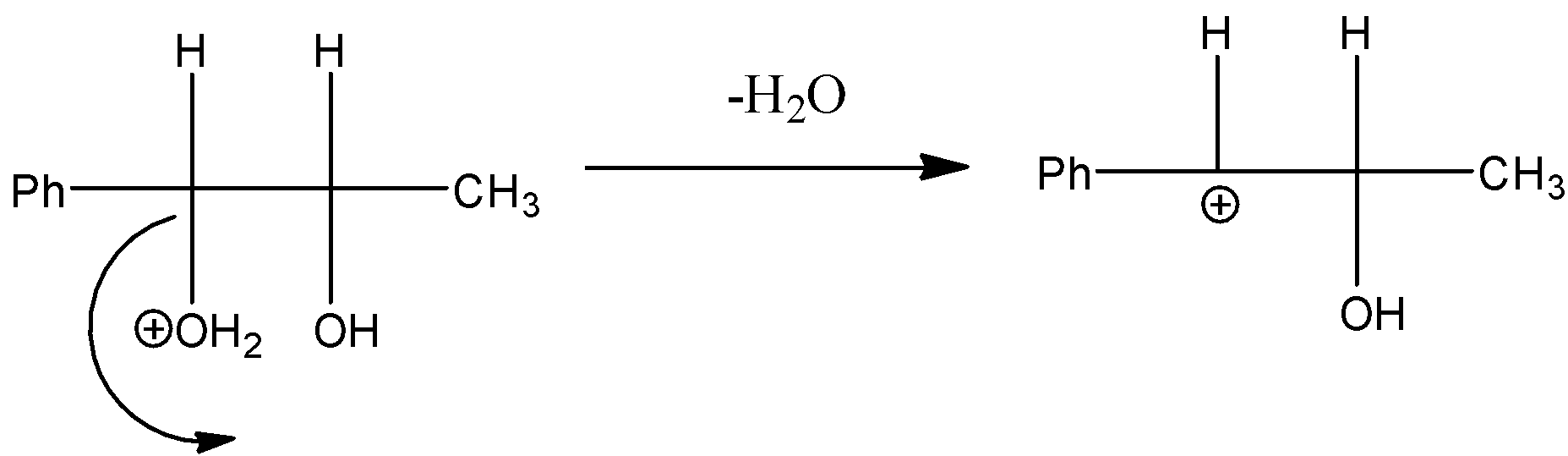
Step 3: The methyl group shifts towards the carbocation in the rearrangement process of the compound.
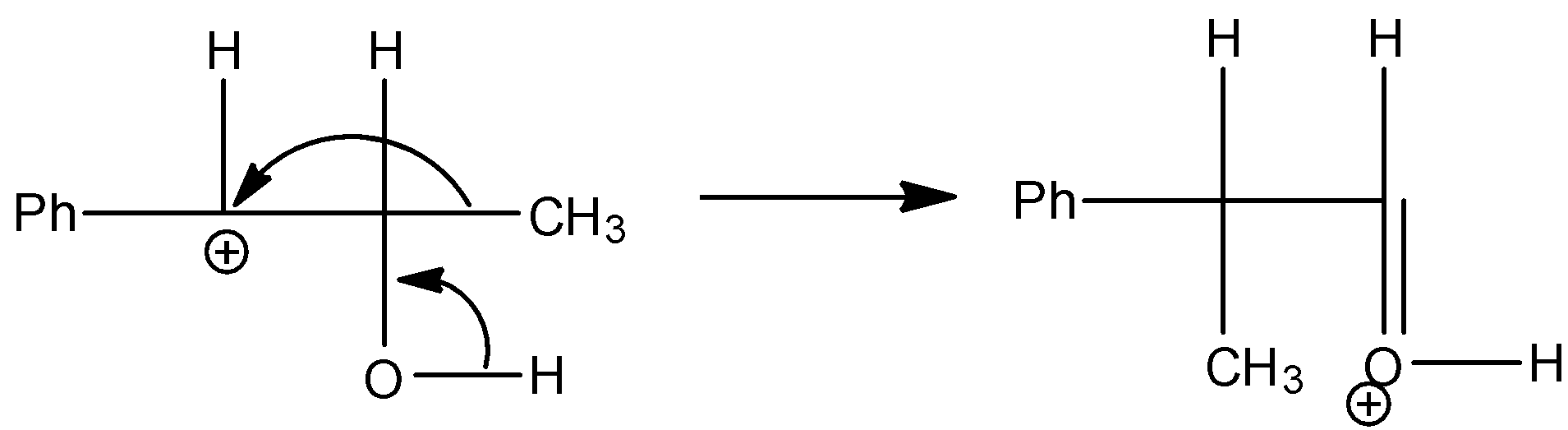
Step 4: The hydrogen which is attached to the oxygen atom which is doubly bonded to the carbon is going to come out and gives the pinacolone as the product.
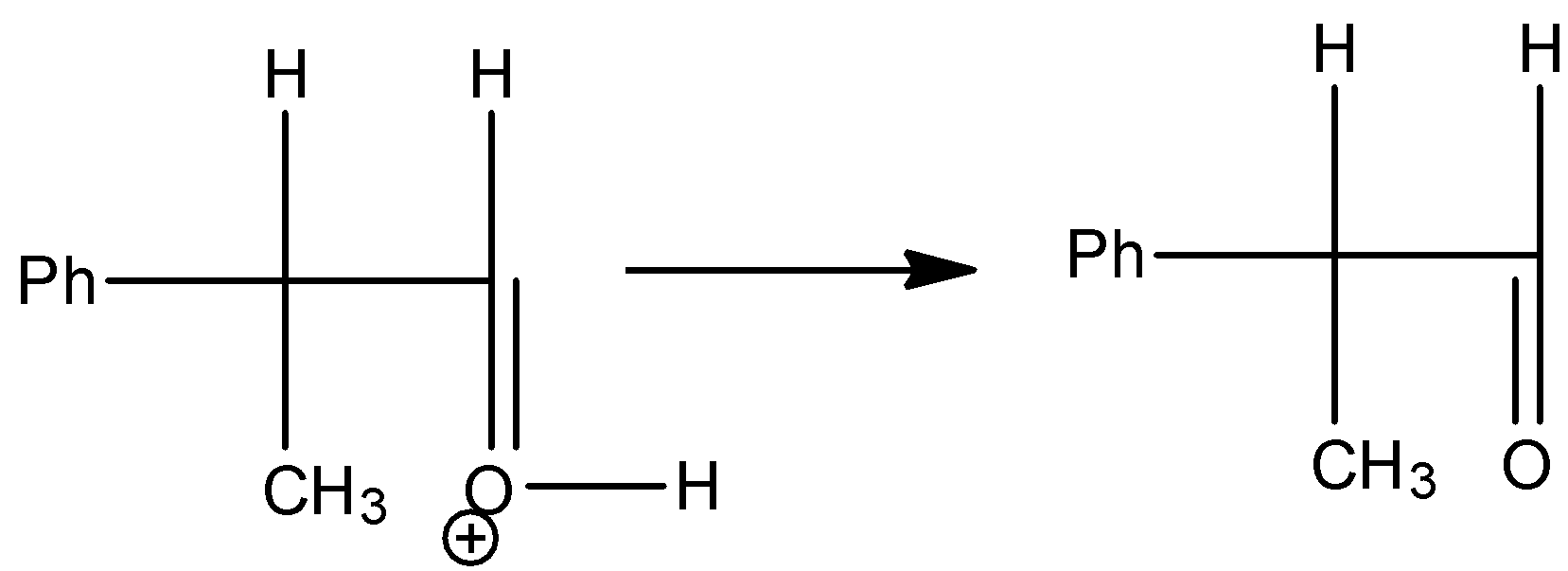
So, we can write the total reaction as follows.
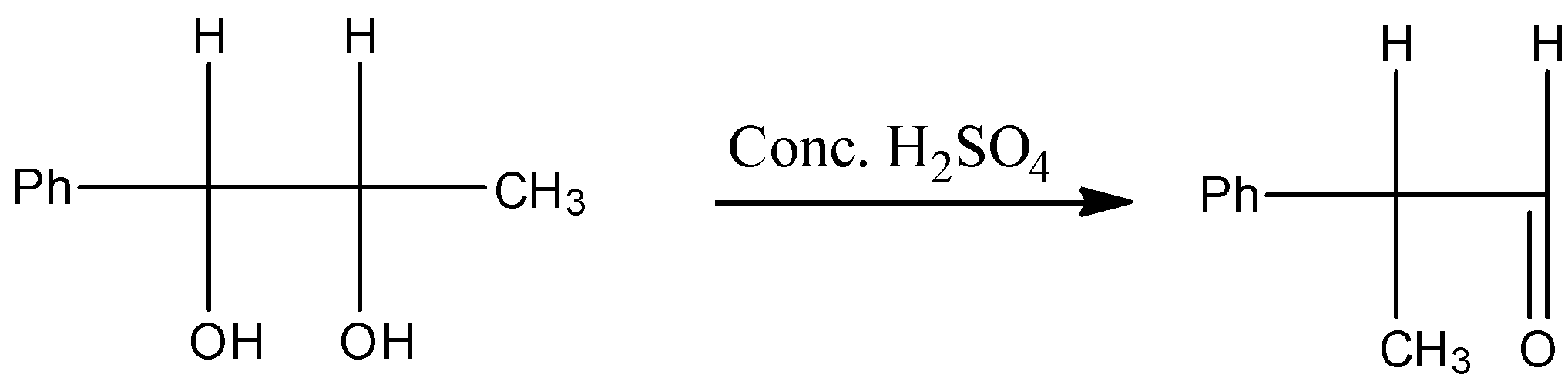
Additional information:
- Pinacol rearrangement is a regioselective reaction.
- The major product is going to form from the rearrangement of the more stable carbocation.
- The pinacol rearrangement is catalyzed by an acid and dehydration of one of the hydroxyl groups happens, which converts the di-alcohols into an aldehyde or a ketone.
Note:
The formation of tertiary carbocation in the given compound is towards the phenyl group attached carbon because phenyl group is an electron withdrawing group it favors the formation of carbocation where it is attached.
