Question
Question: Complete the reaction- 
Solution
In order to answer this question, we need to know the role of each reagent and how it will attack the reactant. Here initially, SO2Cl2 helps in the chlorination of the alkane ring i.e. cyclopentane ring. Then it reacts with Mg in the presence of ether indicating the formation of Grignard reagent.
Complete Step By Step Answer:
Let’s understand the complete mechanism of the reaction.
First, the chlorination of alkane i.e. cyclopentane takes place in the presence of SO2Cl2 , ROOR and light, we get chlorocyclopentane. Then, it undergoes a reaction with magnesium in the presence of the ether solvent, we get a Grignard reagent. Then, when the Grignard reagent reacts with the carboxylic acids, again we get the alkane. But here, there is a difference that deuterium is present in carboxylic acids in place of one hydrogen, so we will get a deuterium attached alkane compound.
Let’s see each step of the reaction one by one so that you can have a better look at how the reactions are taking place.
1. Chlorination of Cyclopentane ring.
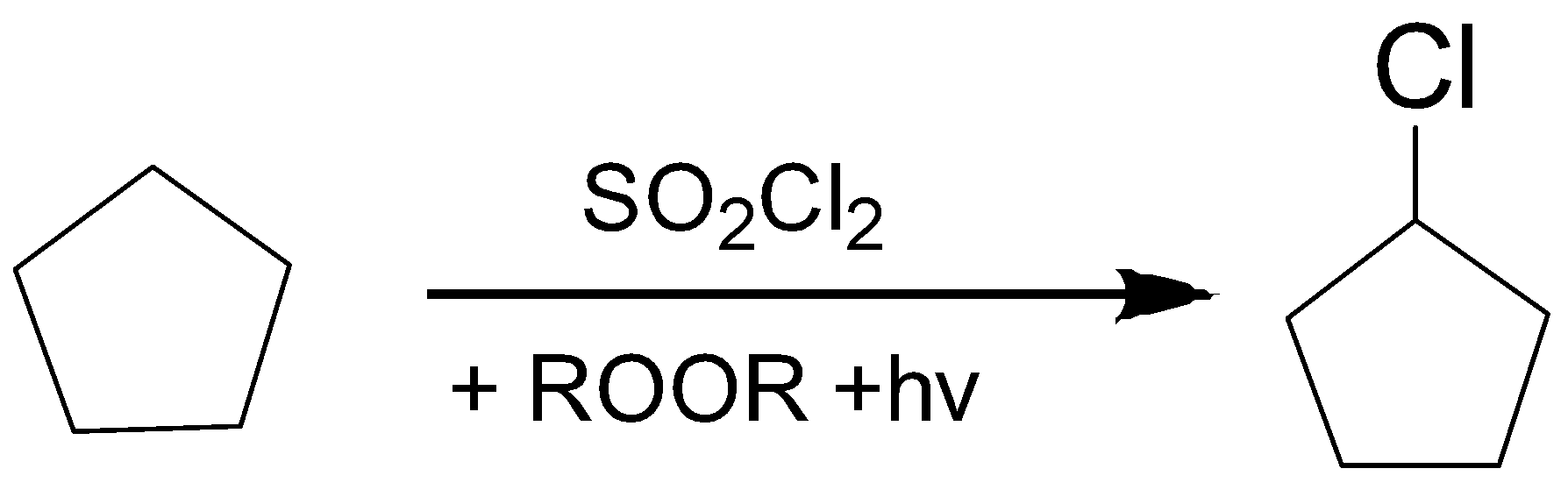
2. Formation of Grignard reagent
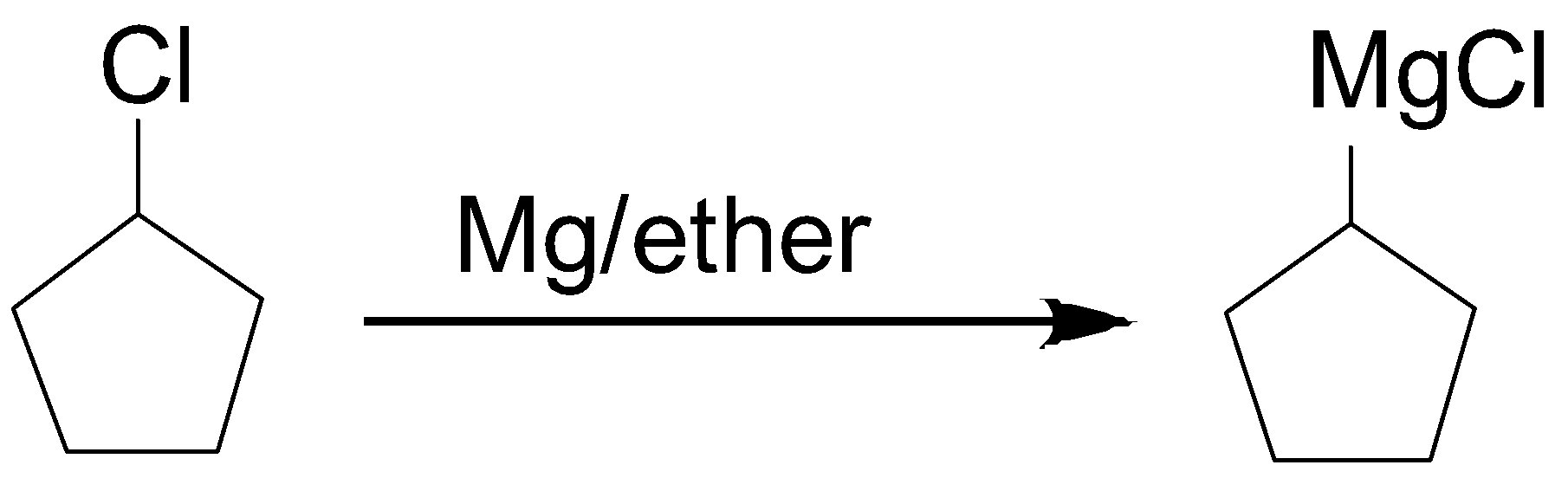
3. Formation of the alkane ring. Here MgCl gets removed and attached with a carboxyl group.
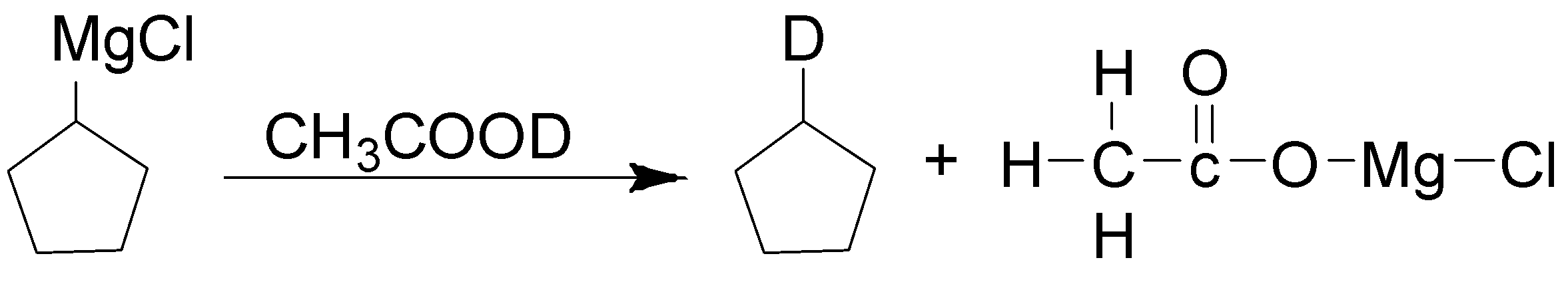
The complete reaction is as follows:
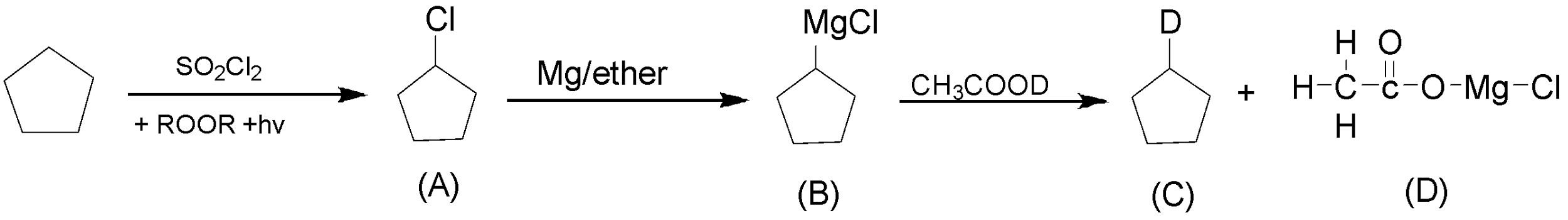
The above reaction shows all the products.
Note:
It must be remembered that the function or we can say the role of each reagent in order to achieve such a type of reaction. Grignard reagent is very important in organic chemistry and it is used in the synthesis of various organic compounds. Also, do not forget to neutralize or stabilize the charges in the reactants or products.
