Question
Question: Complete the reaction: 
Solution
Here, in this reaction, electrophilic substitution is most likely to occur. Electrophilic substitution is basically, a type of chemical substitution reaction in which an electrophile removes the functional group from the compound and attaches itself.
Complete answer:
Now, we can see that here we are asked about the equation in which 2,3−dimethylbut−2−ene is reacting with HI and we need to find out the product formed. Now, such kinds of reactions are electrophilic substitution reactions.
Markovnikov’s rule: so, as per the Markovnikov’s rule, when a protic acid, which in this case, it is HI , attacks an unsymmetrical alkene then the hydrogen atom attaches itself to the carbon on which there are more number of hydrogens and halide atom attached itself to the carbon on which there are more number of alkyl groups.
But here in this case we can see that we do not have any unsymmetrical alkene but instead the alkene present as the reactant is symmetrical, therefore, this rule will not be applied here in this case. The molecule present is symmetrical or planer so it is easy for the electrophile to attach itself to the molecule. We can also see that there we have methyl groups attached, so, as we know methyl groups are electron donating groups so the inductive effect will be high. Therefore, we can say that the methyl groups due to their electron donating nature methyl group will increase the electron density on the alkene groups and this will make alkene more nucleophilic in nature and also the carbocation which will be formed during the reaction will be stabilised due to the electron density.
So, let’s have a look at the product which will be formed when alkene reacts with protic acid:
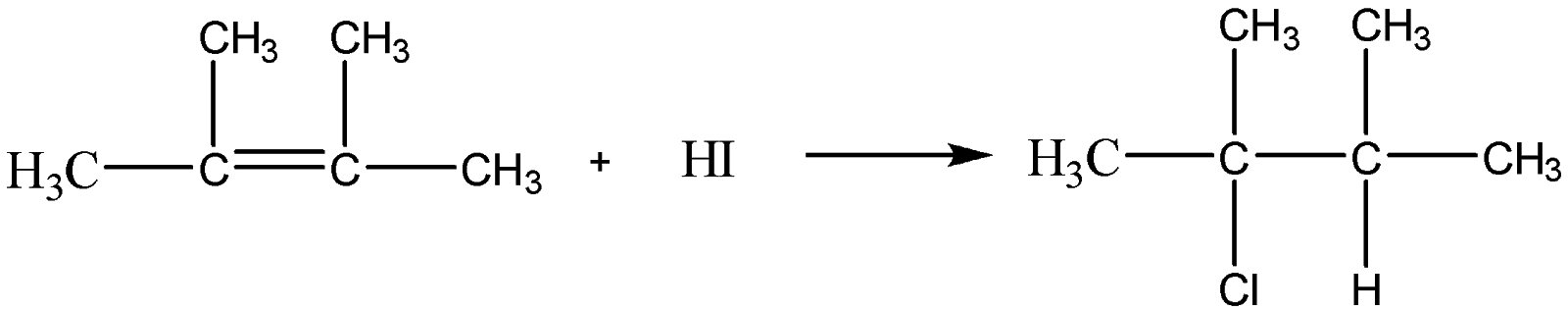
So, 2−chloro2,3−dimethylbutane will be formed as the final product in this reaction.
Note:
Basically, in the electrophilic substitution reaction, an electrophile attacks the nucleophile and forms a product. This is a three step process in which the first one is the generation of the electrophile, as in this case, H is an electrophile then the other step is the formation of the intermediate carbocation and the final step is elimination of the proton from the intermediate which was formed in the second step.
