Question
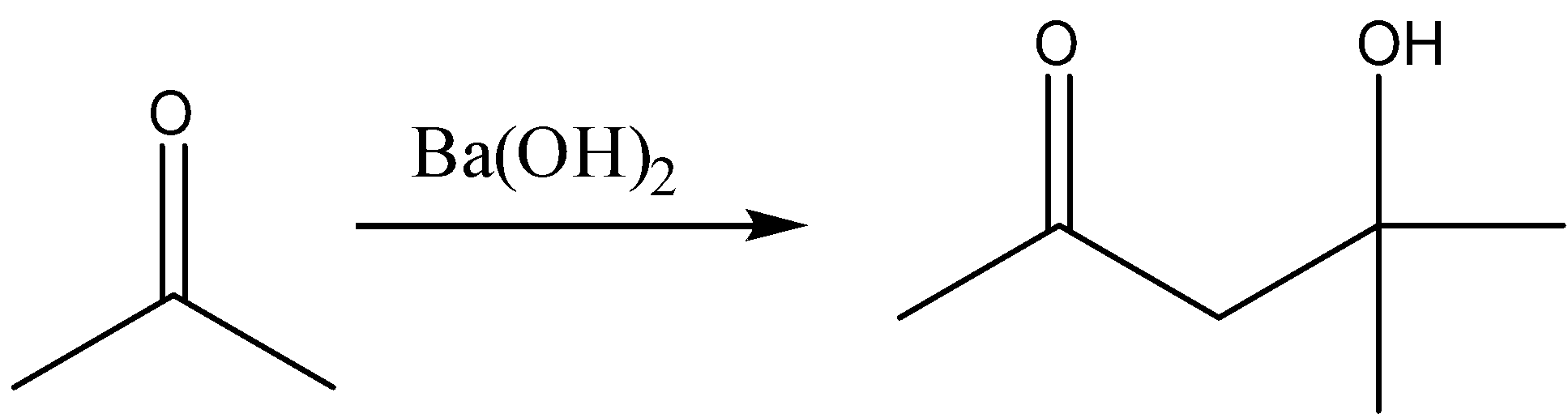
Question: Complete the reaction: \[C{H_3}COC{H_3}\xrightarrow{{Ba{{(OH)}_2}}}?\]...
Complete the reaction: CH3COCH3Ba(OH)2?
Solution
The reaction refers to a condensation reaction between two reactant molecules. In this reaction, new carbon-carbon bonds after combination of both the reactant molecules. There will be elimination of a simple molecule.
Complete step by step answer:
CH3COCH3 is the structure of acetone. Acetone is a common name for propanone. Another name for acetone is dimethyl ketone. The structure of acetone is as follows.
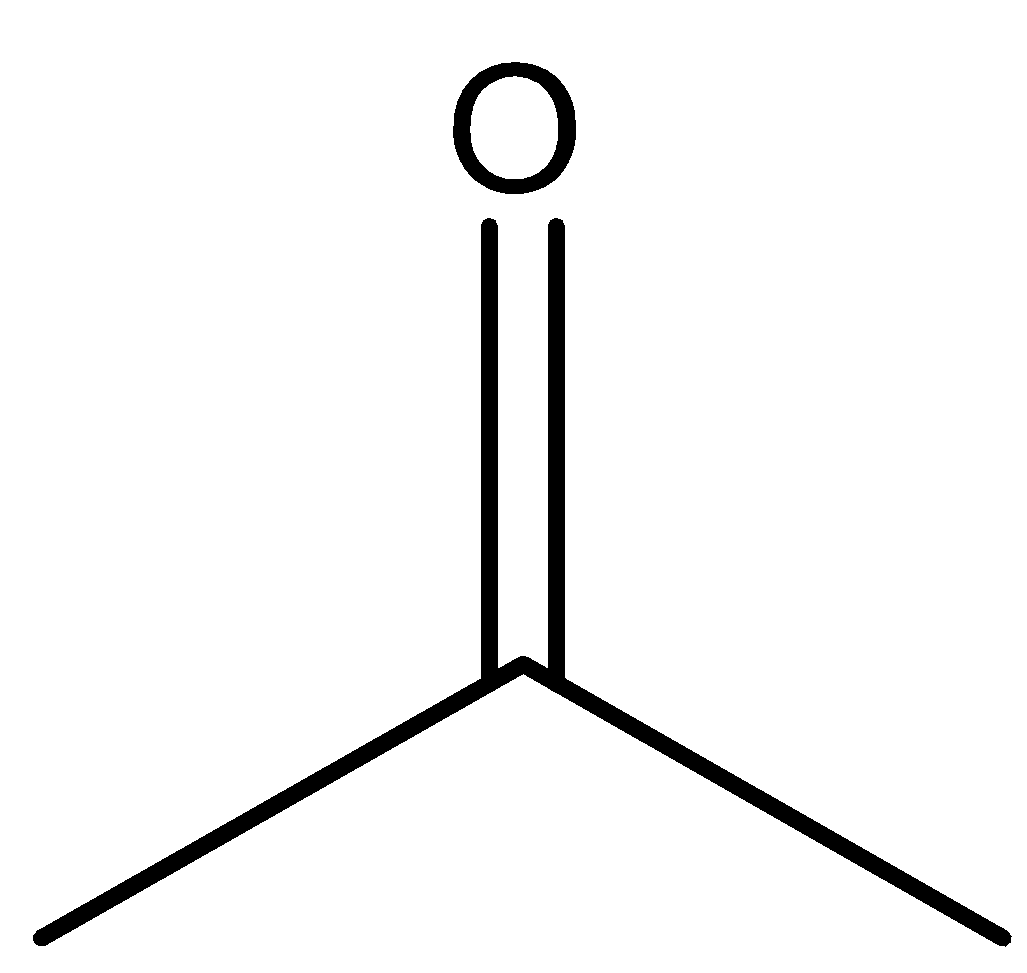
Acetone is used as a solvent in many reactions. Acetone is a volatile solvent which means that it gets evaporated when kept open.
Ketone when reacts with a mild base undergoes self-aldol condensation. For the aldol condensation to take place there should be presence of α- H atoms. In this reaction the hydroxide ions from the base abstracts the proton. This leads to the formation of enol. This enol ion then attacks another ketone molecule at the electron deficient carbon atom of the carbonyl group and thus there is a formation of new carbon- carbon bond. Simultaneously a water molecule will get eliminated. After condensation the product formed will be β-Hydroxy ketone. This product is generally known as Ketol which indicates that it consists of ketone as well as alcohol as functional groups.
When the base Ba(OH)2 is added to the acetone, some of the acetone molecules will undergo enolization and enols will be formed. Base releases hydroxide OH - ions in aqueous solutions. This hydroxide ion will abstract one of the α- H atoms. The reaction will be as follows:
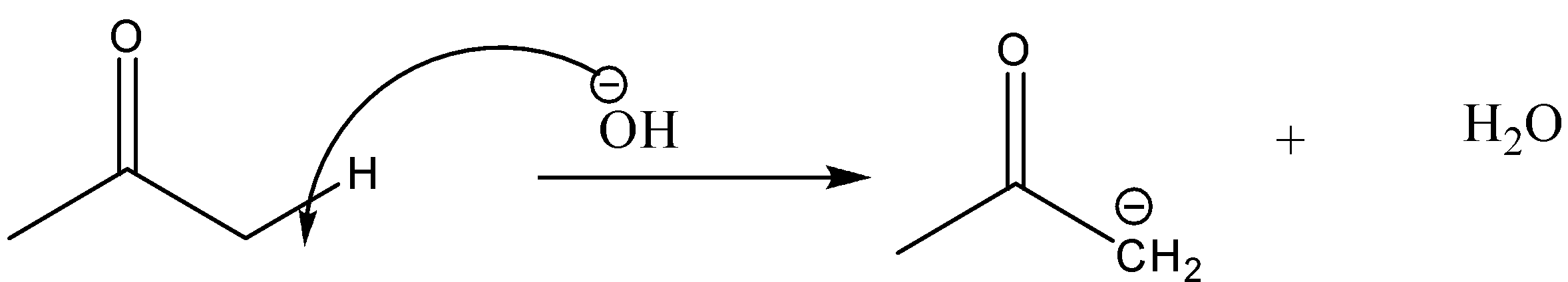
Now, this carbanion will act as a nucleophile and it will further attack the carbon of the carbonyl group of another acetone molecule. The reaction will be:
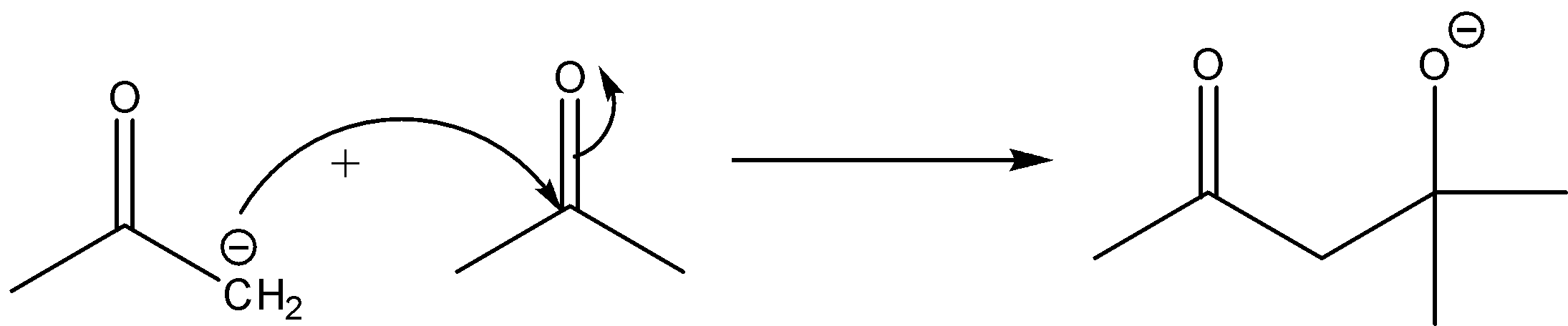
There will be protonation taking place further.
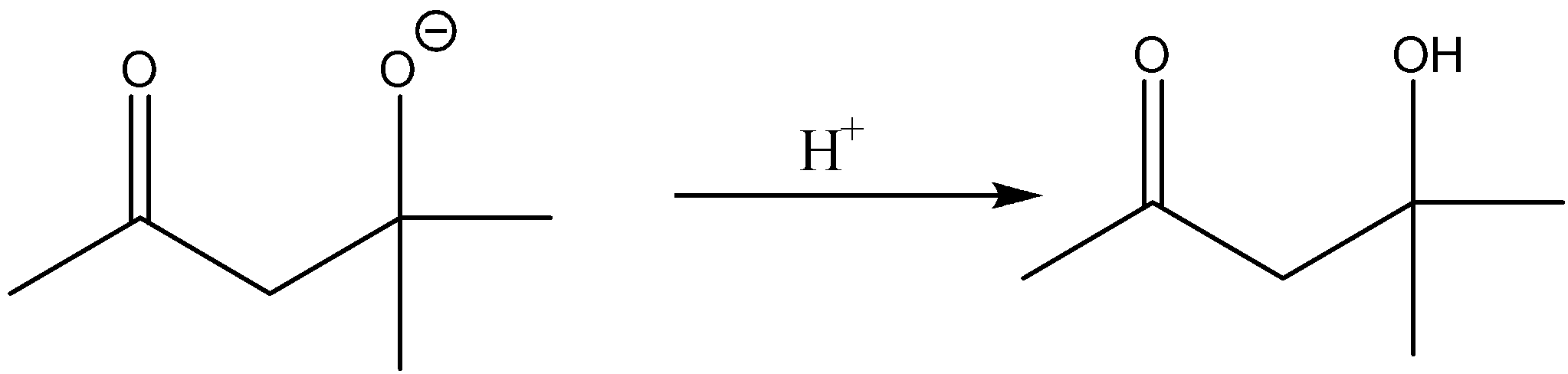
The compound thus formed has 2 functional groups. The product thus formed is β-Hydroxy ketone. To provide an IUPAC name we have to give priority to one of the functional groups. So the keto group gets more priority than the alcohol group. The group which gets more priority should be assigned a low number. So accordingly we have to start numbers from left. There is one methyl group and a hydroxyl group attached to the fourth carbon atom which has to be considered as substituents. Therefore, the IUPAC name given for this compound is 4-Hydroxy-4-methyl-pent-2-one.
The final reaction is:
Note: Carbon of the carbonyl group is not reactive towards electrophile but oxygen of the carbonyl group will be reactive towards electrophile. The condensation reaction can take place between two different carbonyl compounds. That is a condensation between two different aldehydes or two different ketones or between aldehyde and ketone. Such reactions are known as crossed condensation reactions.
