Question
Question: Complete the reaction and find the product (B) in the following reaction;  in the following reaction;
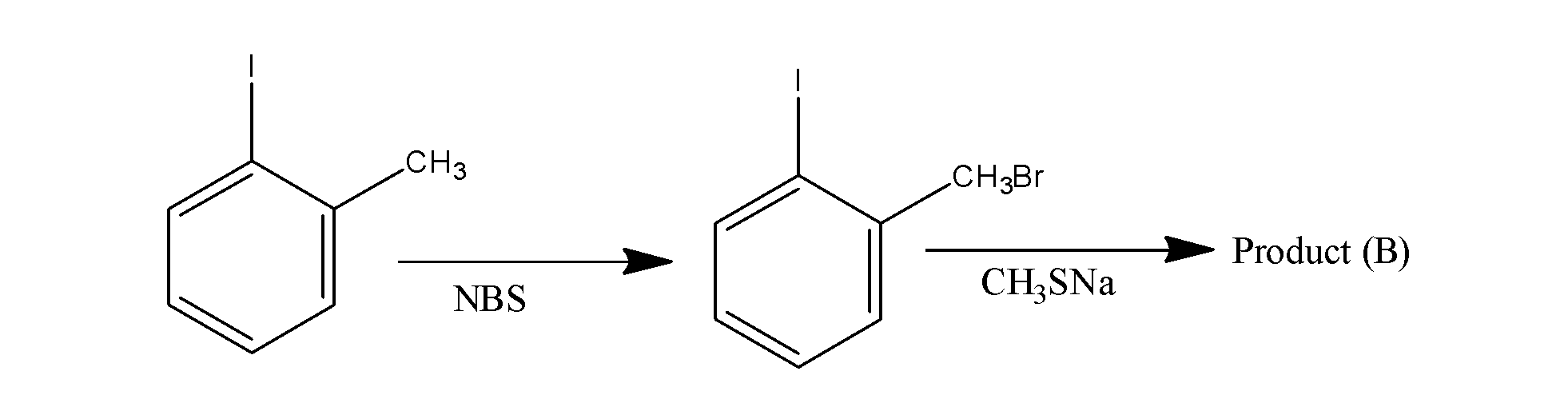
Is:
Solution
When the given reactant, is made to react with the chemical reagent NBS, it results in the formation of the alkyl halide iodobenzene i.e. the alkyl group of the reactant changes to the alkyl halide and when this, alkyl halide iodobenzene is made to react with the next reactant, the halide group leaves forming sodium halide and forms the final product B. now with the help of this you can easily complete the given reaction and find the product. Now solve it.
Complete step by step answer:
Considering the given reaction as;
When 1-iodotoluene is made to react with the NBS , it results in the formation of the compound , the 1-bromomethyl iodobenzene. The reaction is supposed to occur as;
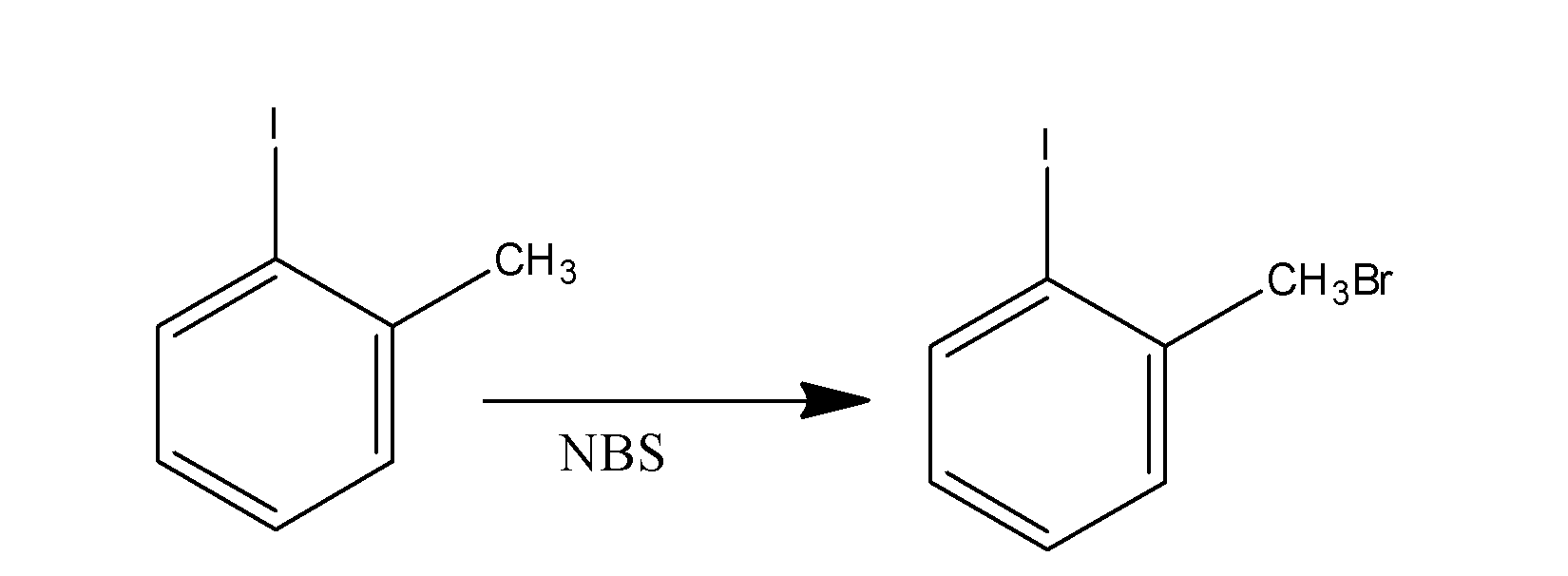
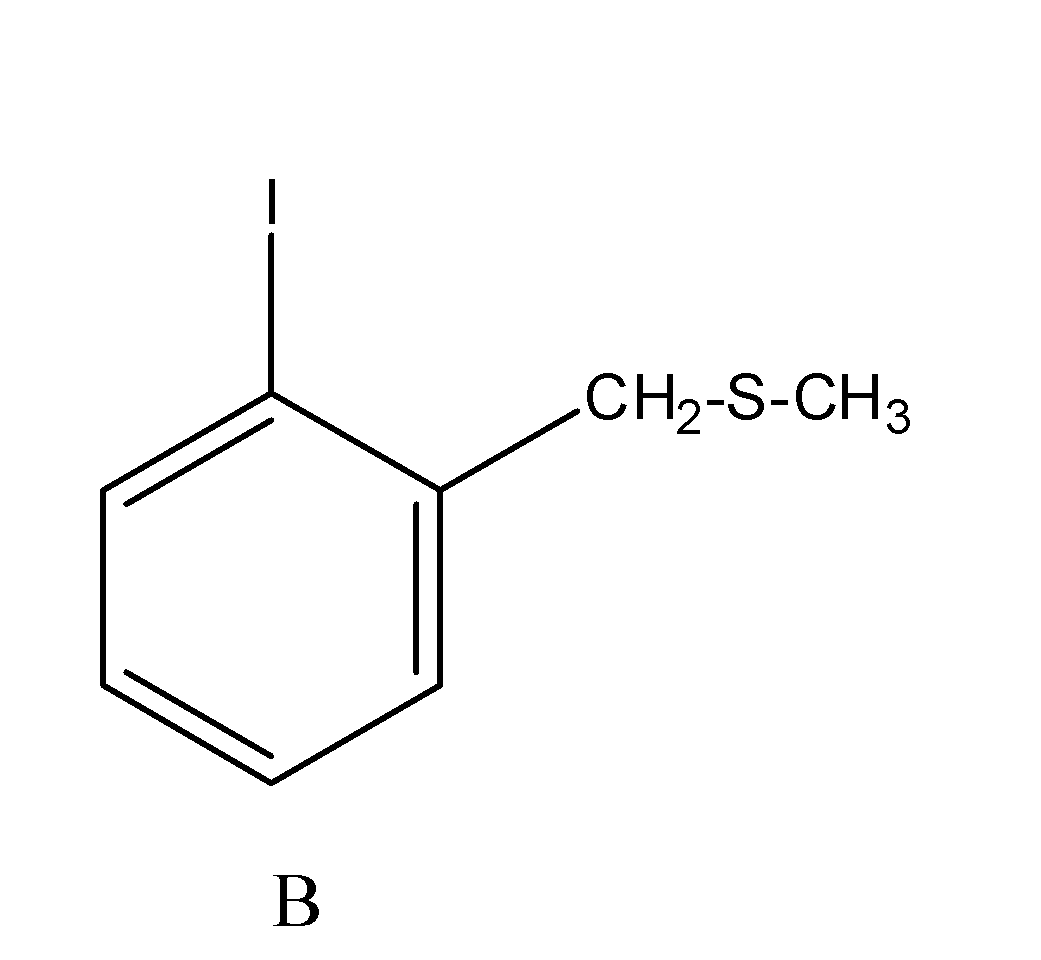
Now when the compound so formed, is made to react with the reagent CH3SNa , then the bromide group of the bromo methyl leaves the methyl group and reacts with sodium to form sodium bromide and the −CH3S group attacks the methyl group and results in the formation of the compound ‘B’. The reaction occur as;
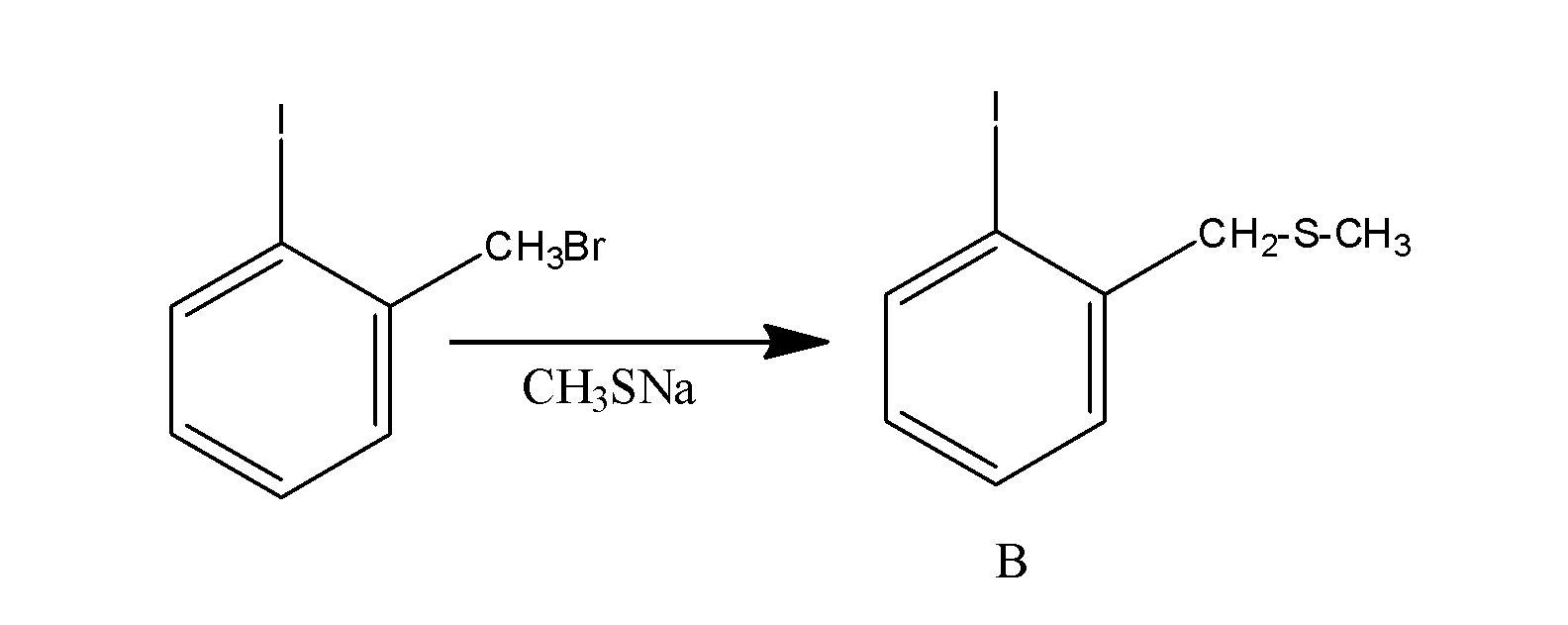
The overall reaction is supposed to occur as;
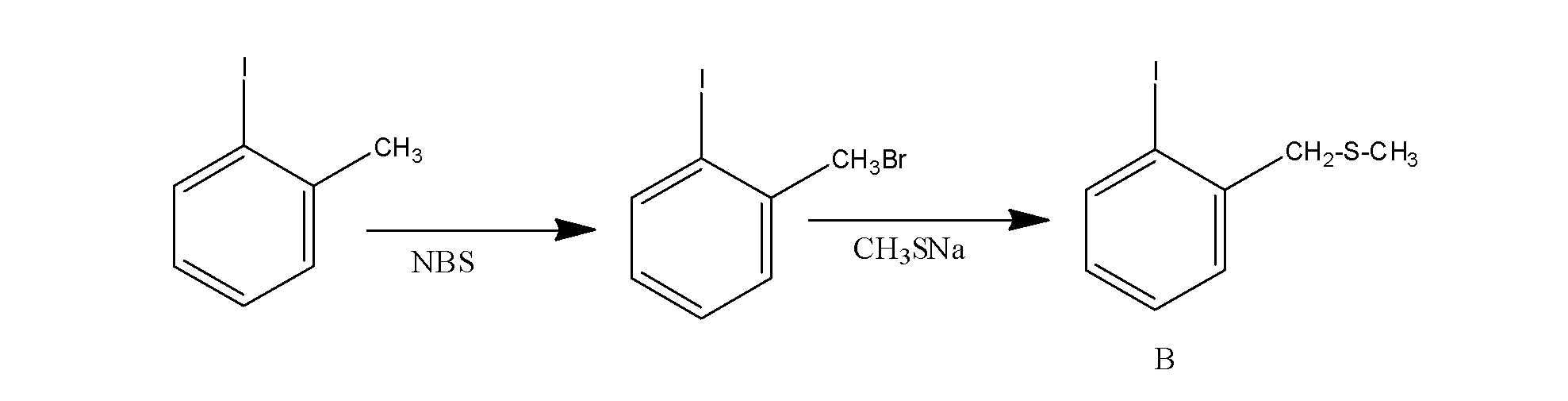
Hence the product (B) is:
Note: NBS stands for the N-Bromosuccinimide. It is a chemical reagent and a source of the bromine free radical and is used as the reagent in the many organic reactions such as the electrophilic addition, electrophilic substitution, radical substitution etc.
