Question
Question: Complete the given reaction: \[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-COOH\xrightarrow[2.Aq.N{{H}_{3}}]{...
Complete the given reaction:
CH3−CH2−CH2−COOH1.Br2,P2.Aq.NH3
Solution
The presence of alpha hydrogen makes the aliphatic carboxylic acid to react with bromine in the presence of phosphorus. This type of chemical reaction is called Hell–Volhard–Zelinsky halogenation reaction.
Complete answer:
- In the question it is asked that butanoic acid is reacting with bromine and later reacting with ammonia.
- We have to find the product in the given reaction.
- The given reaction is as follows.
CH3−CH2−CH2−COOH1.Br2,P2.Aq.NH3
- The reaction contains two steps.
- In the first step the butanoic acid reacts with bromine in the presence of phosphorus.
- In the second step the product formed in the step-1 reacts with ammonia.
Step-1:
- The chemical reaction butanoic acid with bromine in the presence of phosphorus is as follows.
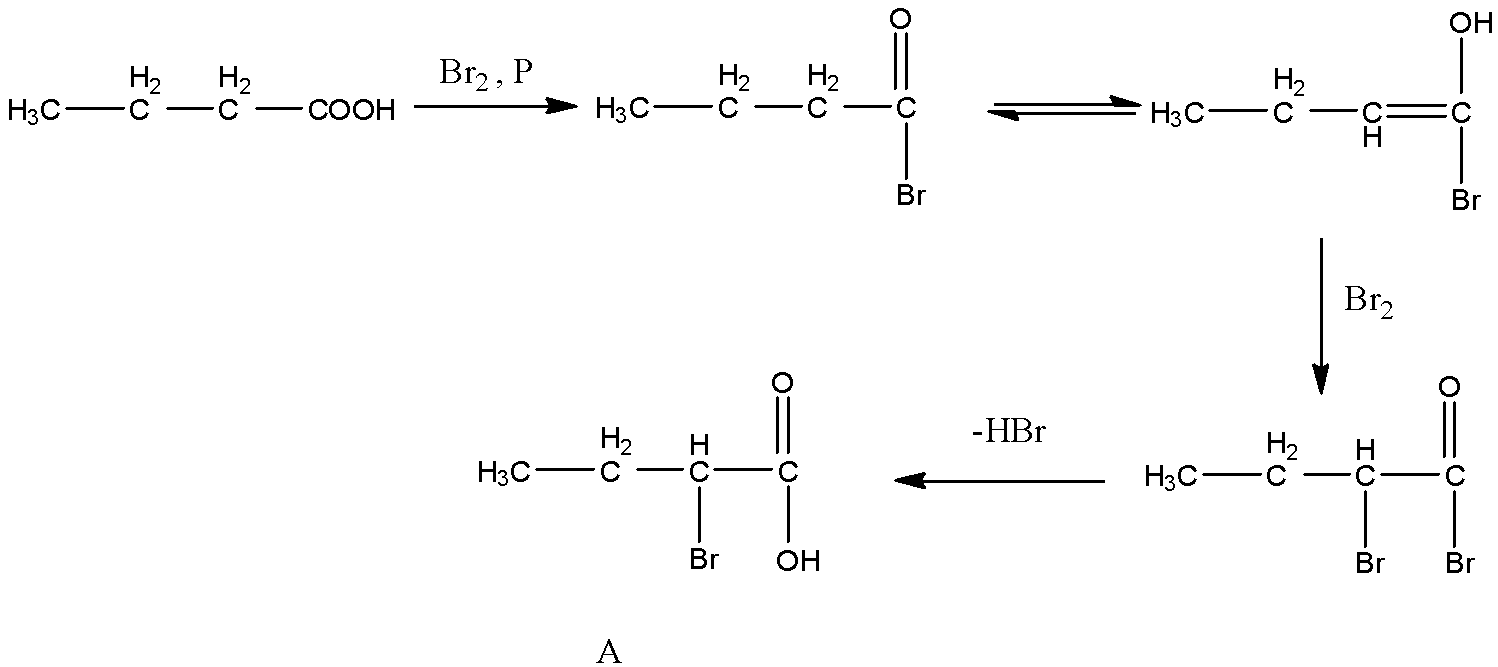
- At starting the carboxylic acid reacts with bromine and forms a bromo derivative. Later there is a generation of unsaturation in the compound due to rearrangement of hydrogen.
- After rearrangement the molecule again reacts with one more bromine and forms a dibromo derivative and later loses hydrogen bromide and gives a product which has bromine at alpha position.
Step-2 - The product formed in step -1 (A) is going to react with Ammonia and forms a product which contains both amine and carboxylic acid functional groups in its structure.
- The chemical reaction of step-2 is as follows.
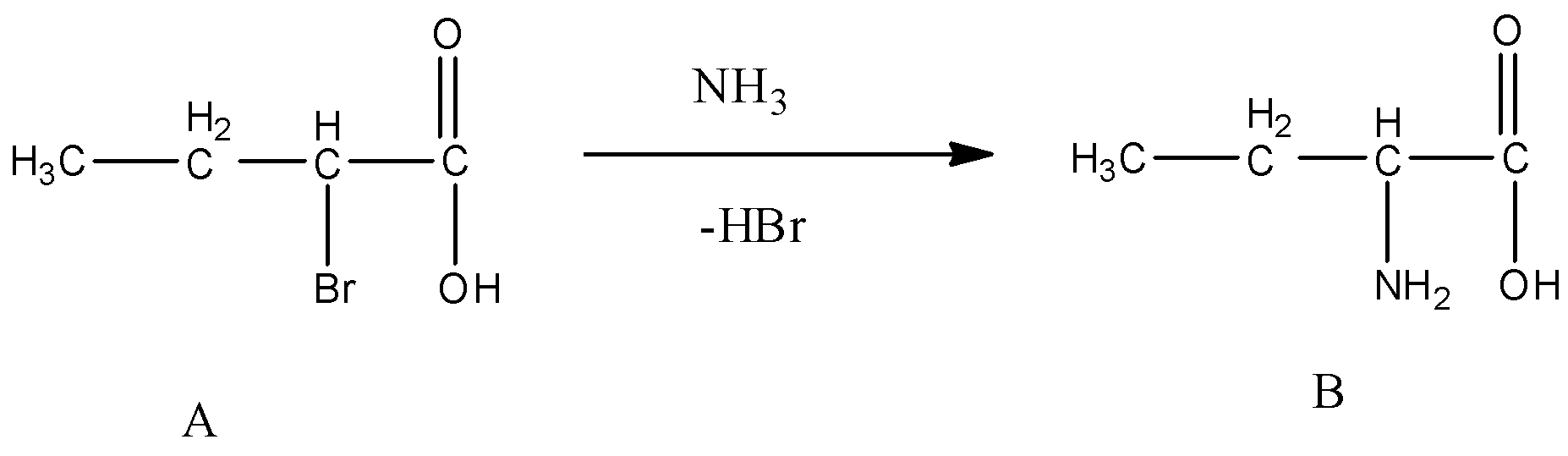
- Finally the product formed contains a amino group at alpha position.
Note: Generally Hell–Volhard–Zelinsky halogenation reaction is used to prepare amino acids. Alanine is an amino acid which can be prepared from propionic acid by using Hell–Volhard–Zelinsky halogenation reaction.
