Question
Question: Complete the following reactions. (a)  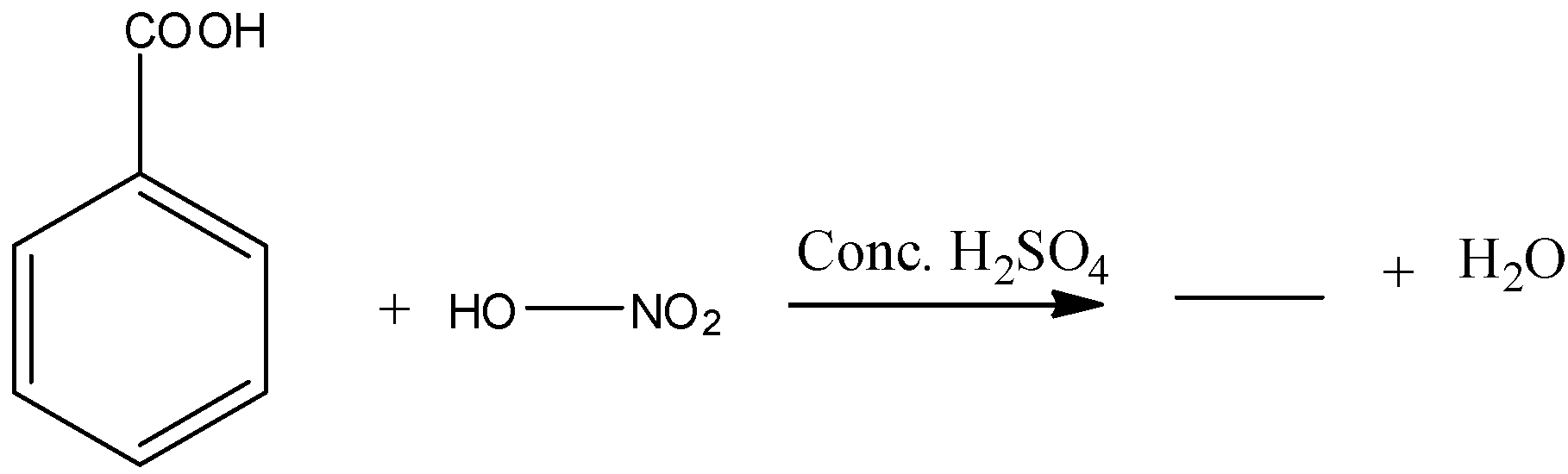
(b) 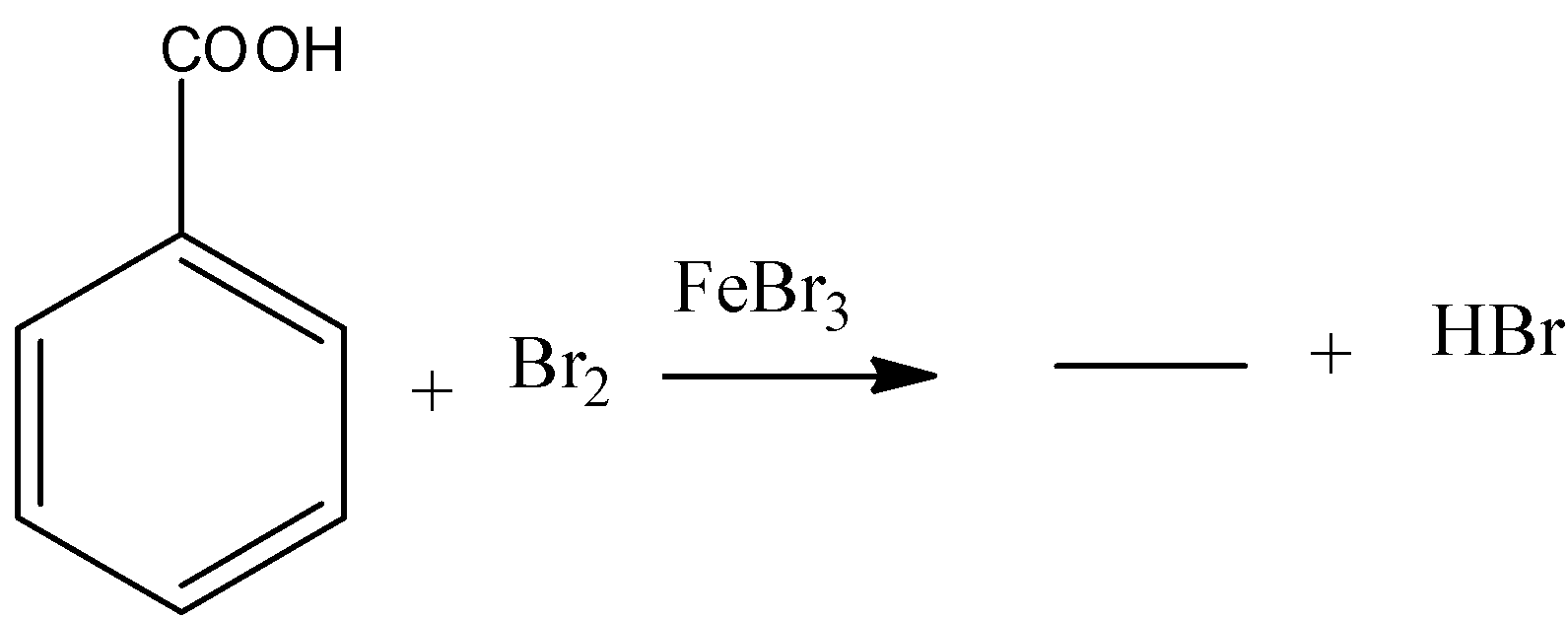
Solution
The presence of the electron withdrawing group directs the reactants to meta position that is why electron withdrawing groups are called meta directing groups. Carboxylic acid comes under electron withdrawing groups and acts as a meta directing group.
Complete step by step answer:
- In the question it is given to complete the chemical reactions.
a) The given chemical reaction is as follows.
- In the above chemical reaction benzoic acid reacts with nitric acid in presence of sulphuric acid.
- The chemical reaction of benzoic acid reacts with nitric acid in presence of sulphuric acid is as follows.
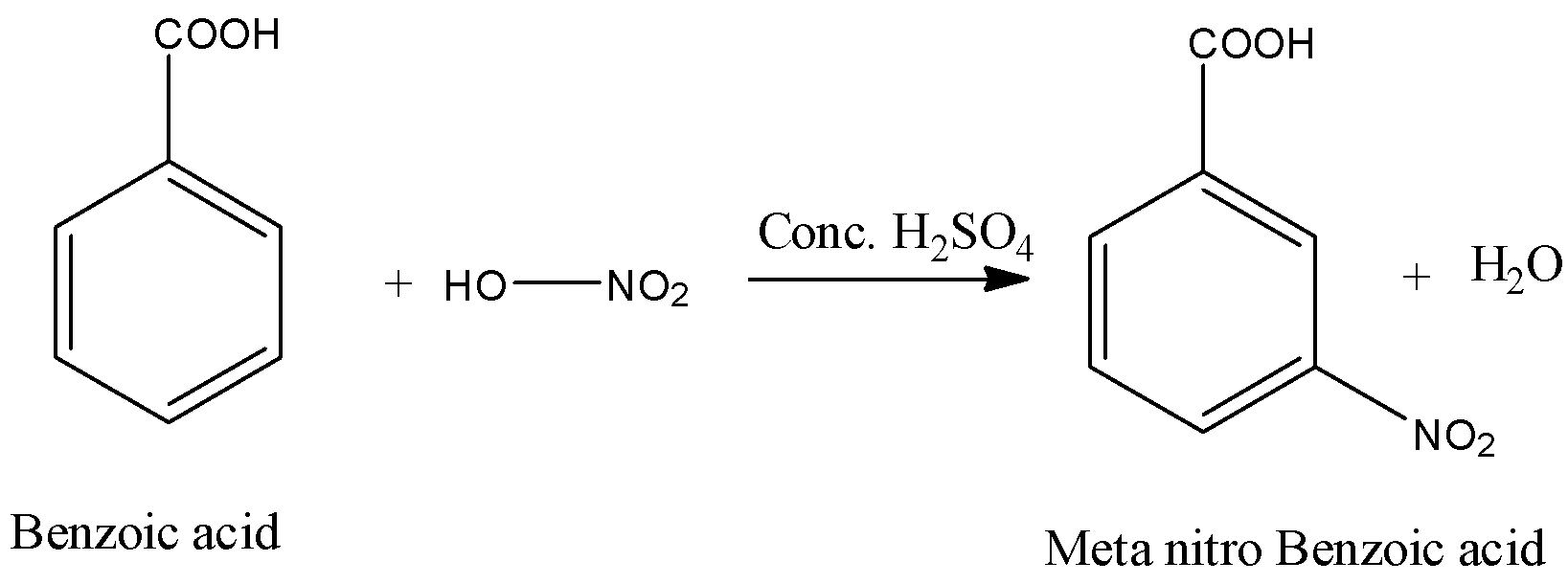
- In the above chemical reaction nitric acid reacts with benzoic acid and forms meta nitro benzoic acid and water as the products.
b) The given chemical reaction is as follows.
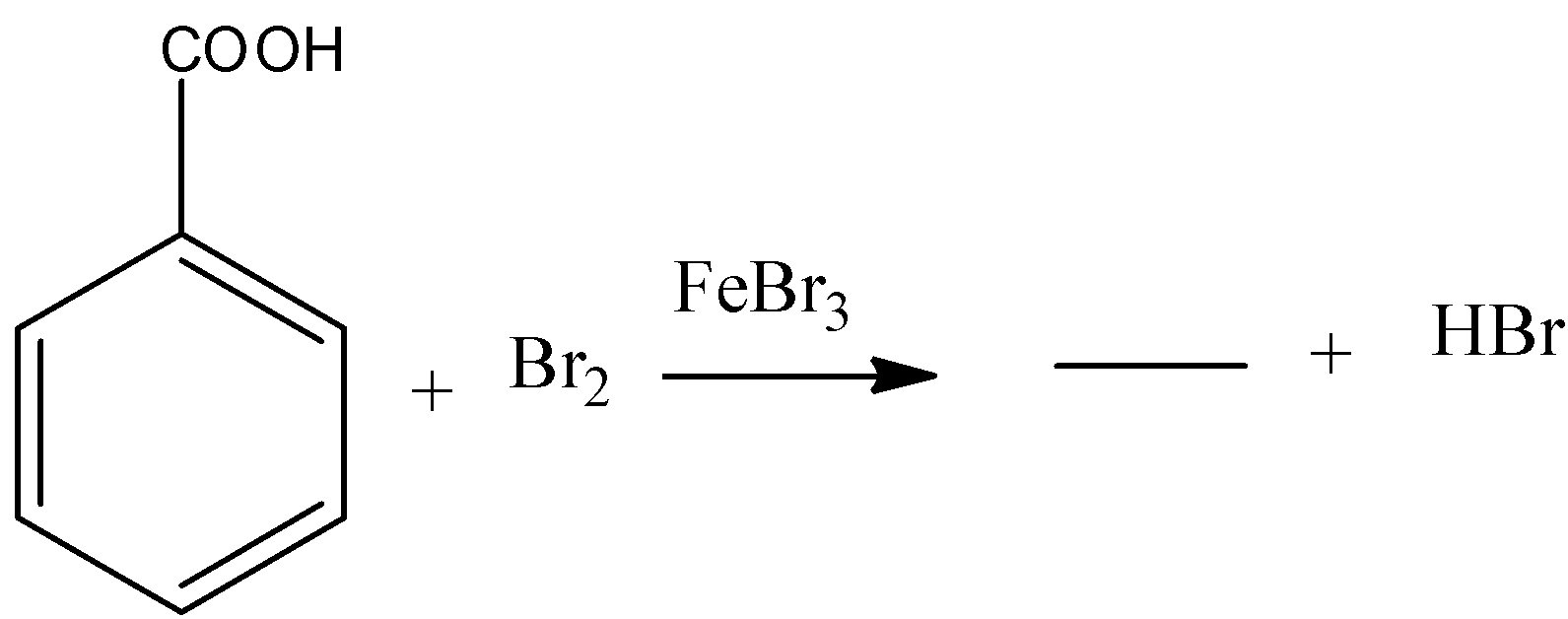
- In the above chemical reaction benzoic acid reacts with bromine in presence of ferric bromide reagent.
- The chemical reaction of benzoic acid reacts with bromine in presence of ferric bromide is as follows
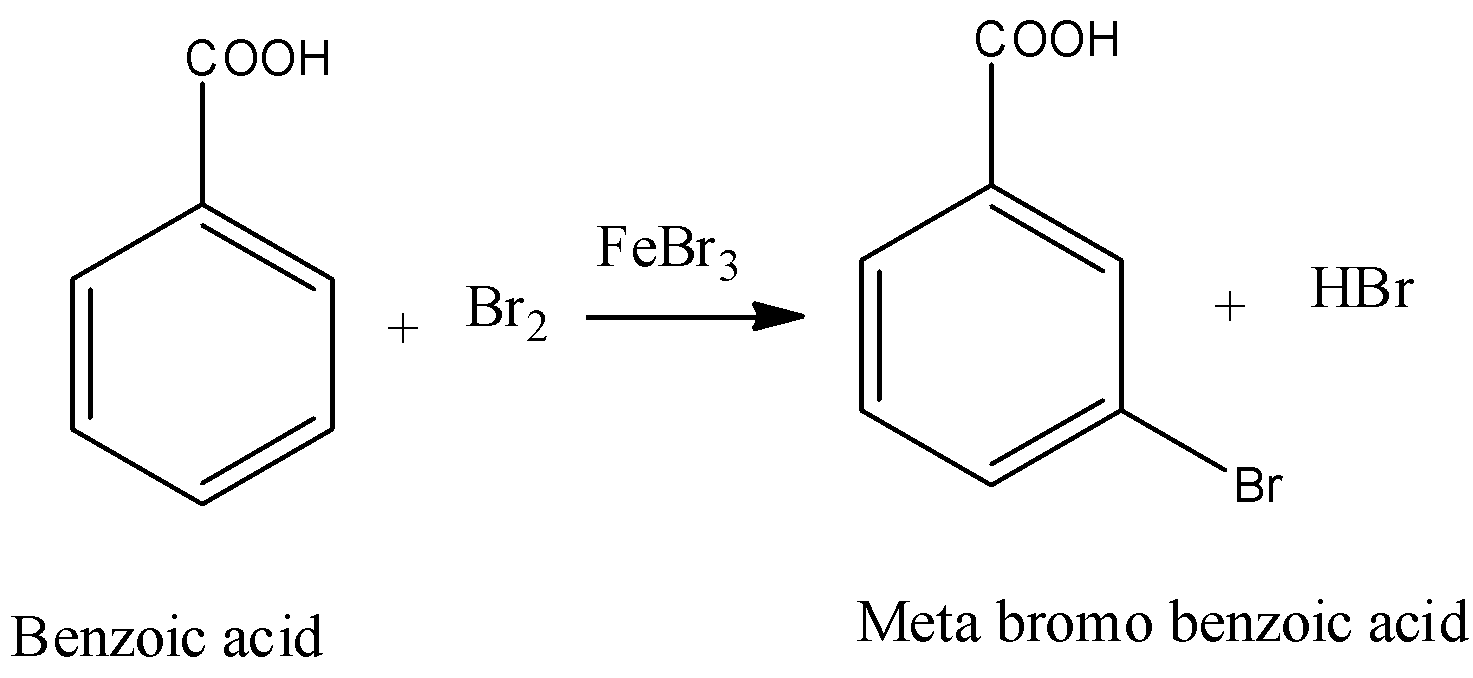
- In the above chemical reaction bromine reacts with benzoic acid and forms meta bromo benzoic acid and hydrogen bromide as the products.
Note: If we are going to add a nitro functional group in the benzene then it is called nitration. If we are going to add bromine in the benzene ring then it is called bromination or halogenation. In presence of the electron withdrawing group in benzene nitration and bromination occurs at meta position in the benzene ring.
