Question
Question: Complete the following reactions? 1\. \( N{a_2}O + {H_2}O \to \underline {} \underline {} \underl...
Complete the following reactions?
1. Na2O+H2O→ ?
2. +→H2CO2 ?
Solution
The substances which participate and chemically change during the reaction are known as reactants whereas those substances which are produced during the chemical reaction are known as products. The representation of a chemical reaction is a chemical equation.
Complete answer:
We will solve it in two steps. The first step includes the reaction of sodium oxide with water. Here Sodium Oxide (Na2O) is a metal oxide which crystallizes in the antifluorite structure. Second step includes the formation of formic acid (H2CO2) which we can obtain by the process of hydrolysis.
Step-1: 1.Na2O+H2O→
As we know, Sodium Oxide is a compound whose chemical formula is Na2O . It is used in glasses and ceramics. When this compound reacts with water H2O , it gives sodium hydroxide. This compound is the basic anhydride of sodium hydroxide.
The complete chemical reaction is:
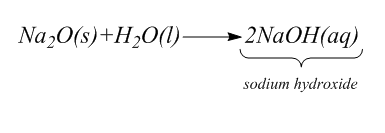
Step-2: 2.+→H2CO2
For this part we have to synthesize formic acid (H2CO2) . For this, first we will use carbonylation of methyl alcohol to form esters.
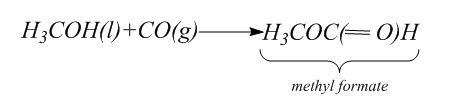
Then we will hydrolyze the methyl formate to form the formic acid and methanol. Hydrolysis is the process in which the bond between the elements of the compound break. Thus, in hydrolysis, addition of water breaks one or two chemical bonds.
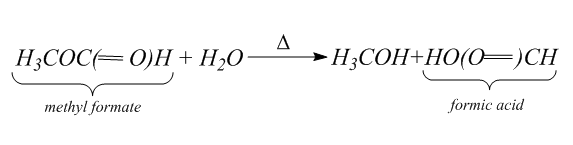
Hydrolysis is the reverse of the condensation which is the process of joining two molecules into a larger one and eliminates a water molecule.
Note:
Formic acid is also formed by the reaction of methyl formate with ammonia. Hydrolysis of methyl formate required a huge excess of water so some routes use the reaction of ammonia with methyl formate to form formamide which on hydrolysis with sulfuric acid gives formic acid.
