Question
Question: Complete the following reaction- 
A.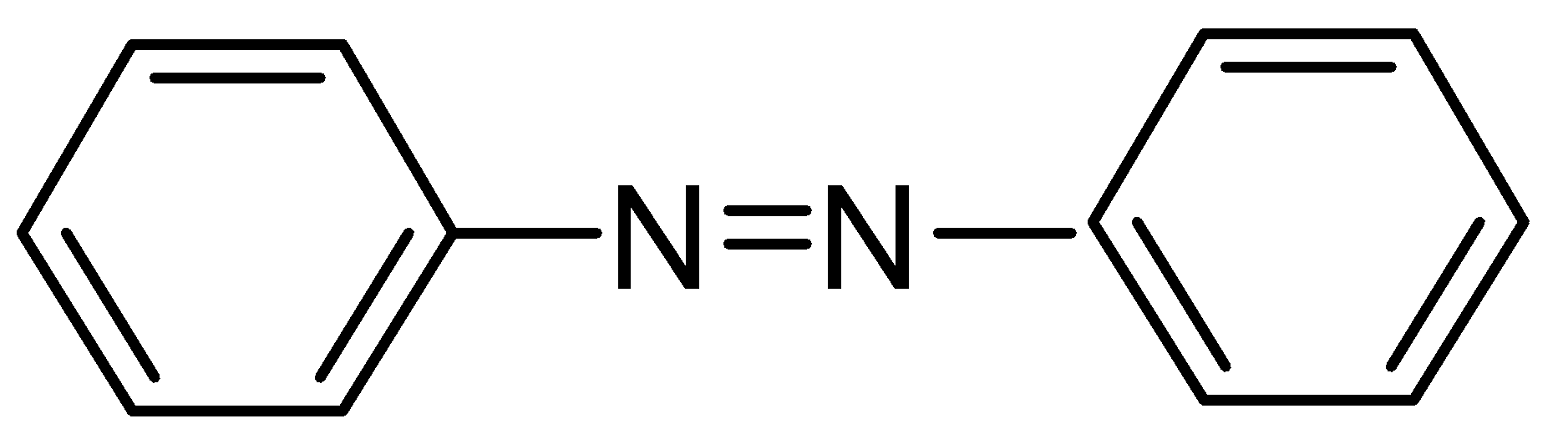
B.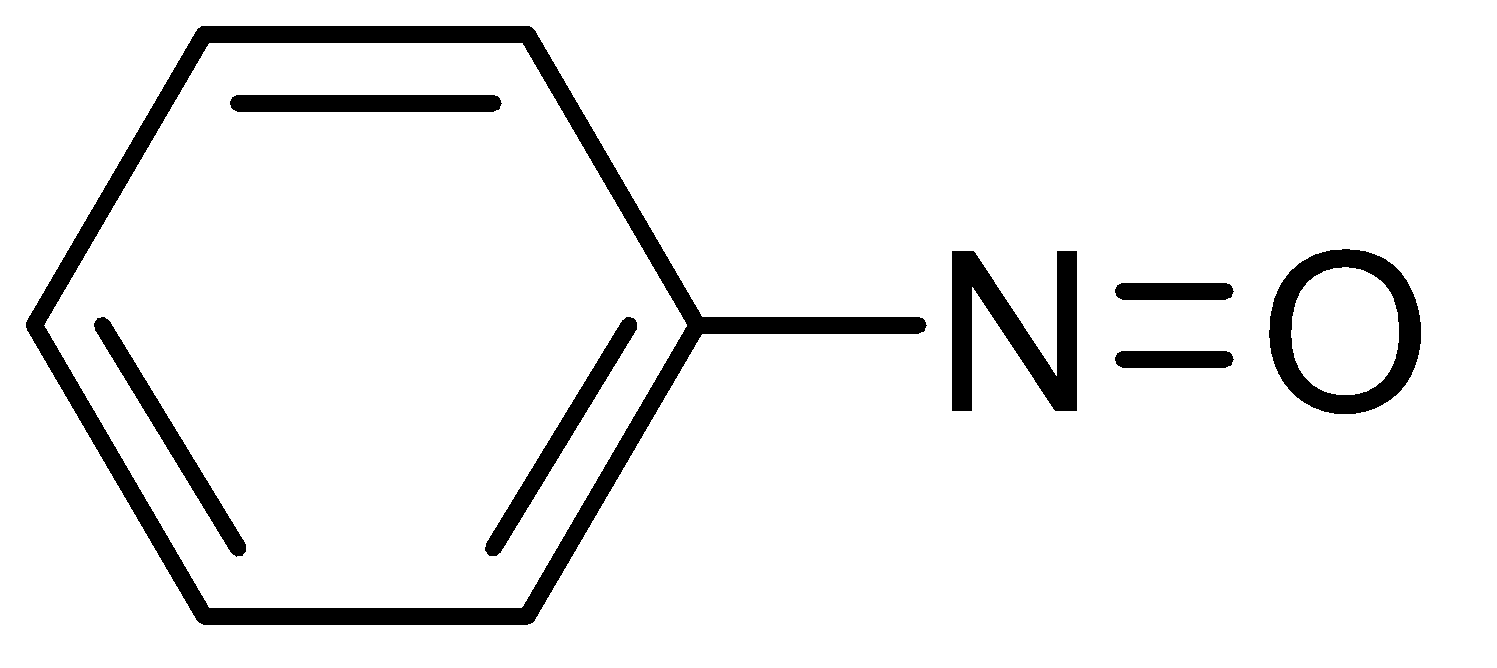
C.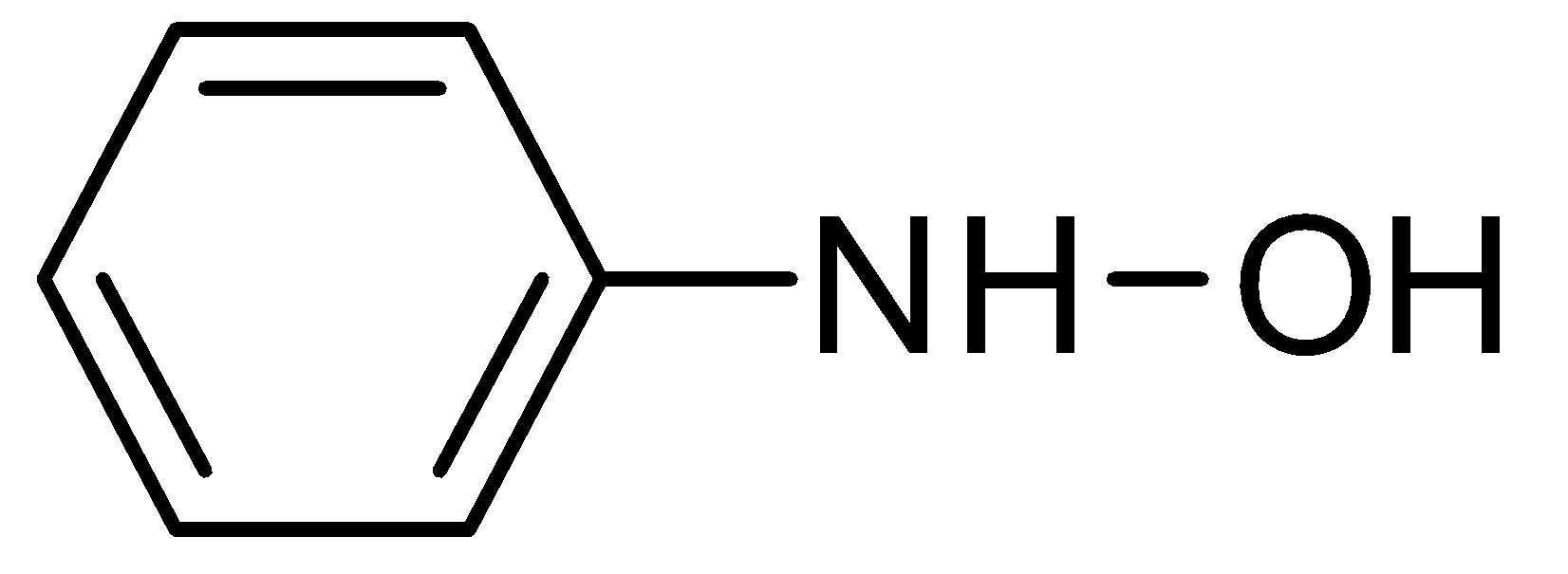
D.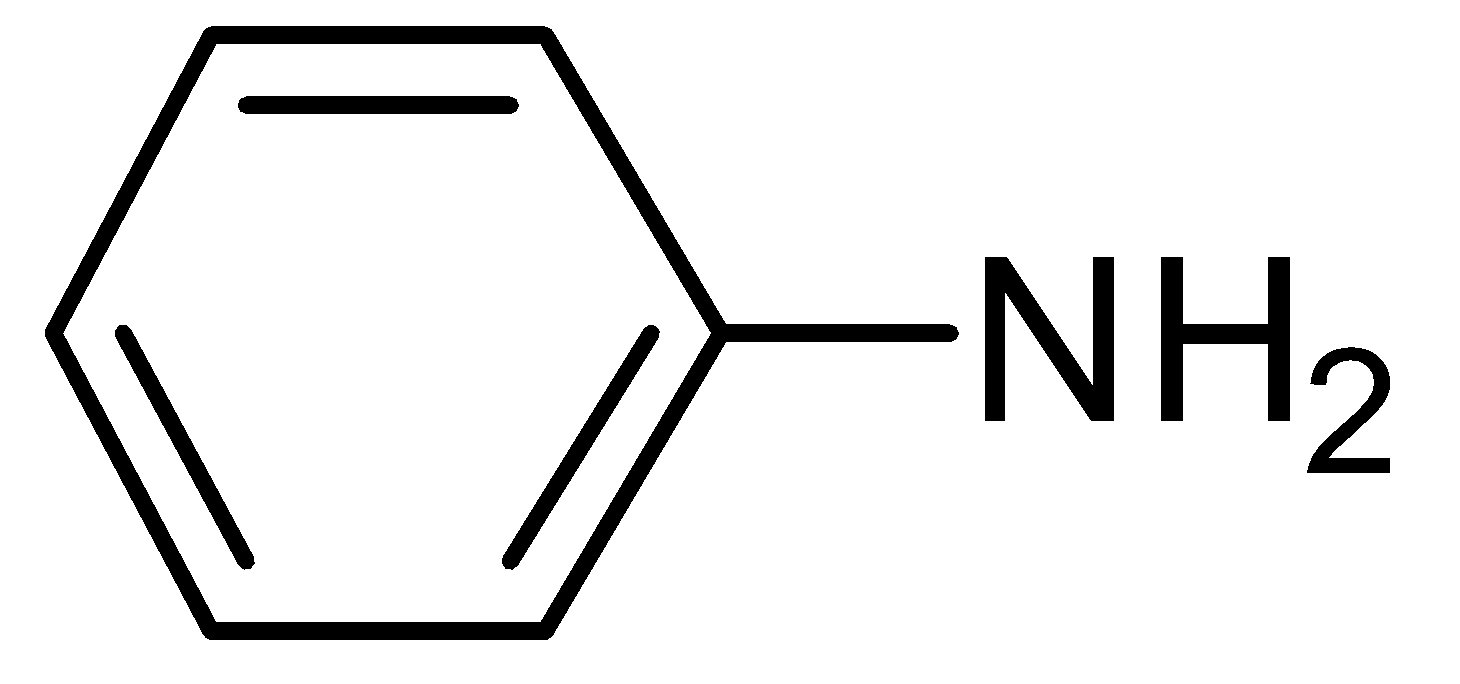
Solution
In the above question, the reagent given is LiAlH4, which acts as a reducing agent. So, we have to find out the product after the reduction of nitrobenzene.
Complete answer:
Lithium Aluminium Hydride is an inorganic compound which is for the reduction of many organic compounds. Its chemical formula is LiAlH4 and it is abbreviated as LAH. It looks like a grey solid. It is used as a nucleophilic reducing agent for the synthesis of organic compounds. It is a highly reactive compound since the bond between Aluminium and Hydrogen (Al−H) is weaker and less stable. It is considered as the best reagent to reduce the polar bonds like C=O, but it cannot reduce multiple bonds like C=C . It is used to reduce aldehyde to primary alcohol, ketone to secondary alcohols, carboxylic acids and esters to primary alcohols, epoxides to alcohols, amides and nitriles to amines and lactones (cyclic esters) to diols and many more. It has wide application in organic synthesis.
Structure of LiAlH4
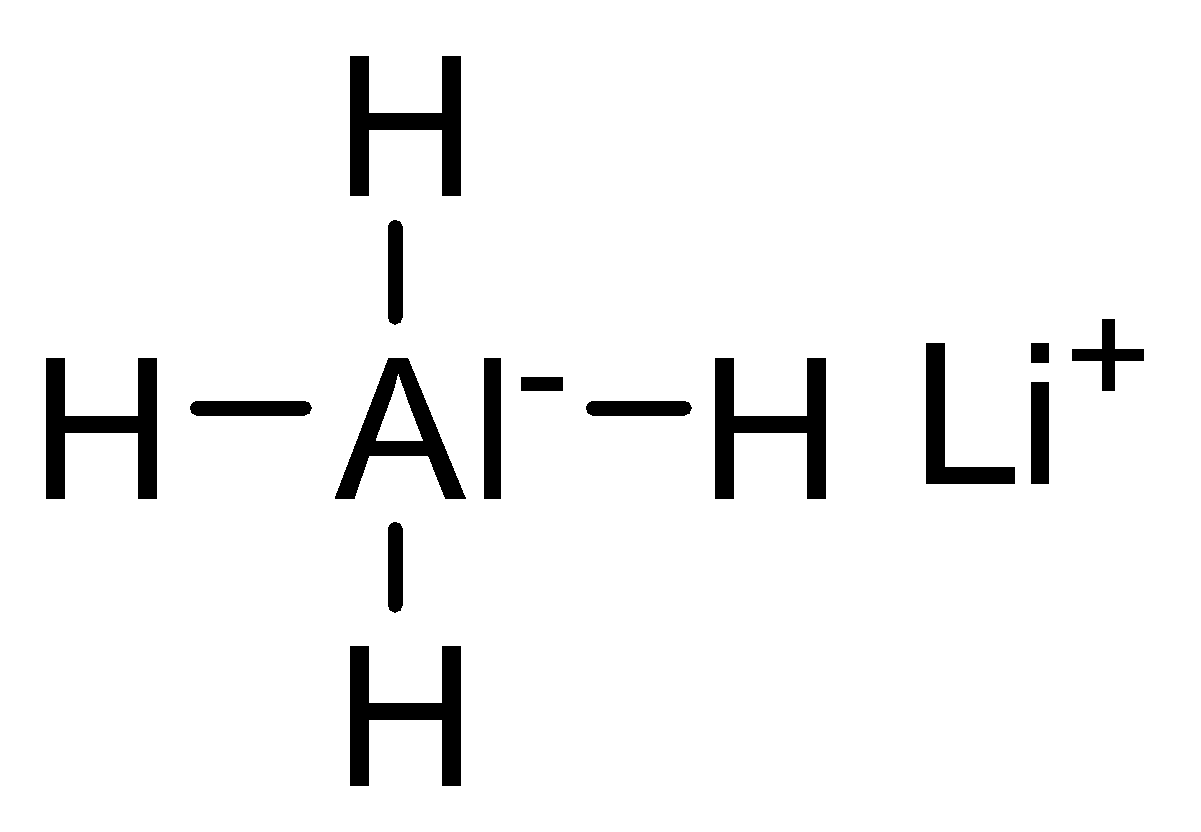
When the reduction of nitrobenzene occurs in the presence of a reducing agent LiAlH4, it leads to the formation of Azobenzene. Azobenzene is a parent compound of a family called aromatic azo compounds. It consists of two phenyl rings linked by a two phenyl linked by (N=N) double bond. These compounds are not thermally stable but they give intense colour and are used as dyes.
Structure of azobenzene:
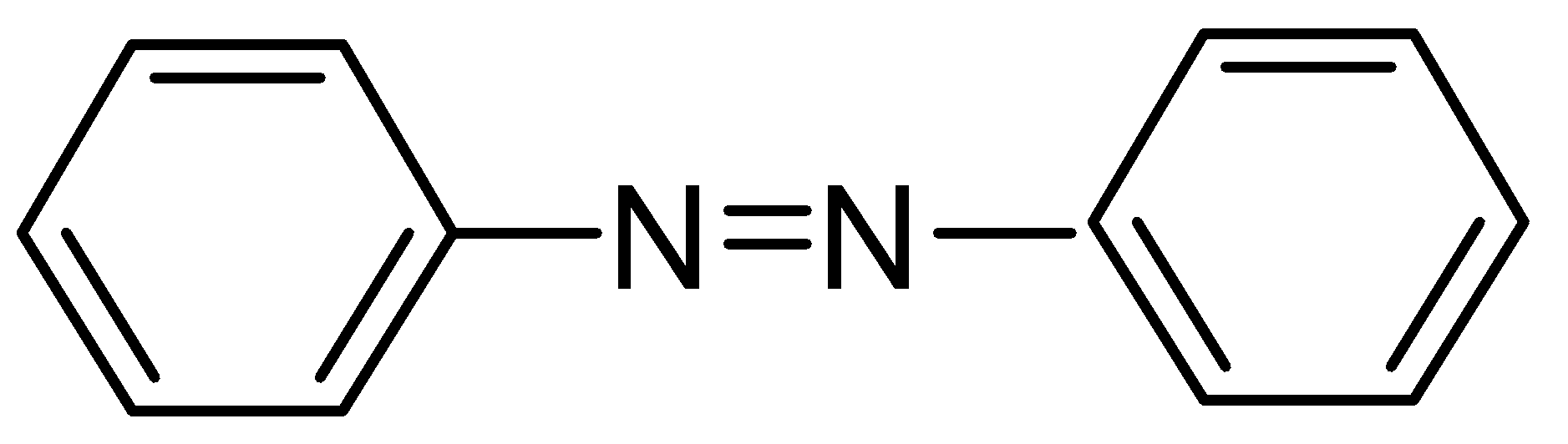
Hence, the correct answer is option (A).
Note:
Nitrobenzene can also be reduced to aniline but this reaction occurs only in the presence of Sn and concentrated HCl and aqueous NaOH.Nitrobenzene can also be converted into N- phenylhydroxylamine in the presence of Zinc and Ammonium Chloride (NH4Cl) in sufficient water and when N- phenylhydroxylamine is oxidized by sodium dichromate (Na2Cr2O7), it gives nitrosobenzene.
