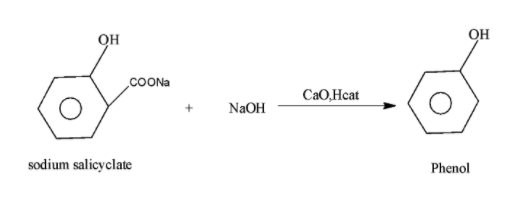
Question
Question: Complete the following reaction: 
Solution
In the given reaction ,the reactant sodium salicylate on reaction with the soda lime in the presence of the heat results in the removal of carbon dioxide molecule from the reactant and such reaction is called the decarboxylation reaction. Now identify the product.
Complete step by step answer:
-The above reaction is of the phenols. So, first of all let’s discuss the phenols. Phenols are the hydroxyl derivatives of the hydrocarbons in which the hydroxyl group -OH is directly attached to the carbon atom of the aromatic ring.
-Phenols are colourless, crystalline solids or liquids and have characteristic phenolic odour and are sparingly soluble in water but completely soluble in alcohols, ethers etc. and their boiling point is also very high.
-They are more acidic than the alcohols and turn blue litmus red and react with alkali metals and alkalis to form their salts. But phenols are weaker acid than the carboxylic acids and therefore, they do not react with the sodium carbonate and sodium bicarbonate.
Now, considering the statement.
-In the above given statement, the sodium salicylate when on reaction with the soda lime i.e. NaOH and CaO in the presence of heat undergoes decarboxylation reaction i.e. removes the carbon dioxide and results in the formation of the phenol along with the removal of sodium carbonate. The reaction occurs as.
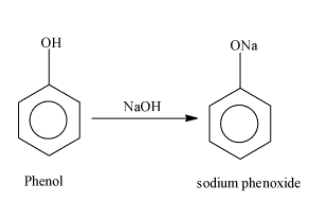
Note: Sodium salicylate used in the above reaction is formed as; First of all, phenol reacts with sodium hydroxide and results in the formation of the compound sodium phenoxide. The reaction occurs as;