Question
Question: Complete the following reaction equations: (a) \(PC{{l}_{5}}+4{{H}_{2}}O\text{ (excess)}\to \) (...
Complete the following reaction equations:
(a) PCl5+4H2O (excess)→
(b) 2F2+2H2O→
Solution
When phosphorus pentachloride is reacted with the water, it results in the formation of the acid and on the other hand, when water is reacted with fluorine, it results in the formation of the acid halide. Now you can easily complete these reactions with the help of it. Solve it.
Complete Solution:
We will discuss the reactions one by one as:
(a) In this reaction, when the phosphorus pentachloride is made to undergo reaction with the excess of water, it forms the acid i.e. phosphoric acid along with the formation of hydrogen chloride. The reaction is supposed to take place as:
PCl5+4H2O (excess)→H3PO4+5HCl
(b) In this reaction, when fluorine is treated with water, it results in the formation of the acid halide i.e. hydrogen fluoride along with the formation of the oxygen gas. The reaction is supposed to take place as;
2F2+2H2O→4HF+O2
Additional information:
Phosphoric acid is an inorganic compound and is also known as the orthophosphoric acid . It is weakly acidic in nature and consists of three -OH bonds attached to the phosphorus and one oxygen atom which is doubly bonded to the phosphorus atom. The structure of phosphoric acid is as;
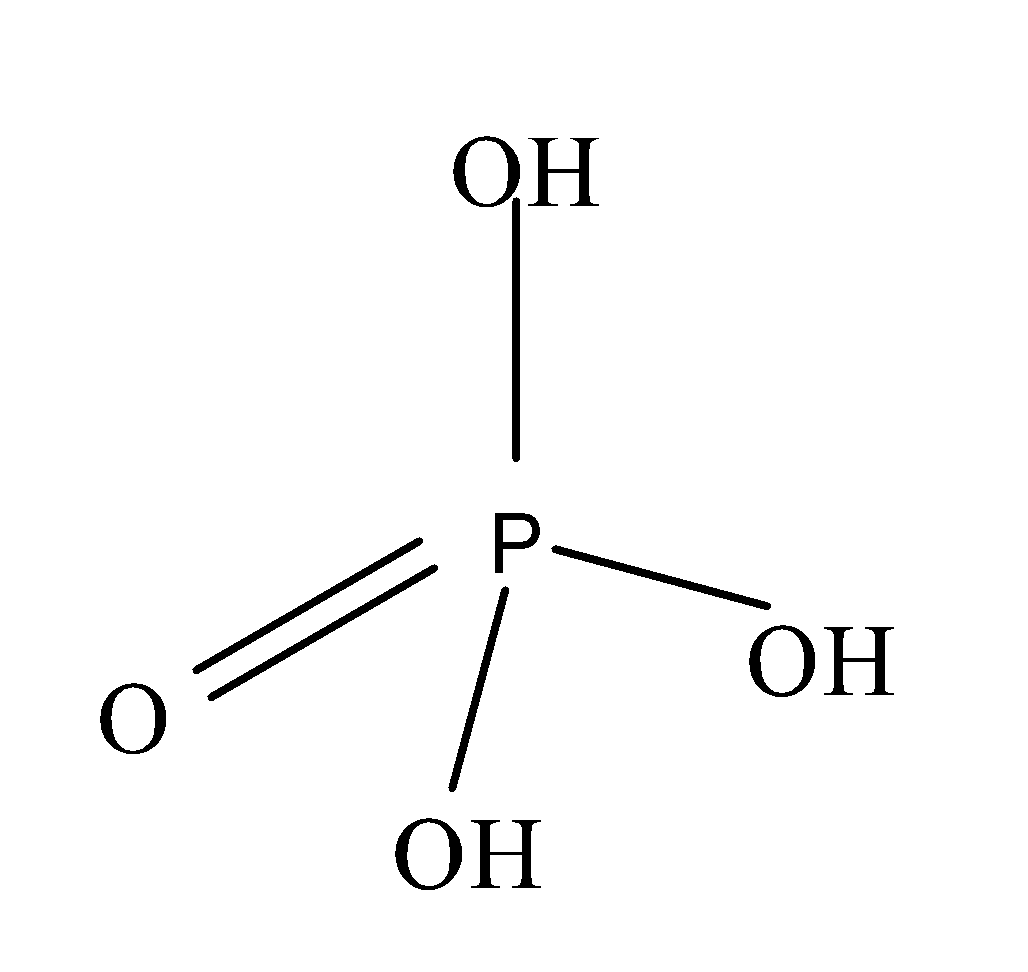
Note: Phosphoric acid is triprotic acid. It is so because it consists of three -OH bonds and we know that oxygen atom is more electronegative in nature and when this phosphoric acid is dissolved in water, it easily releases the three acidic hydrogen atoms and thus, is triprotic in nature.
