Question
Question: Complete the following reaction equation : A. \[{C_6}{H_5}{N_2}Cl + {H_3}P{O_2} + {H_2}O \to \] ...
Complete the following reaction equation :
A. C6H5N2Cl+H3PO2+H2O→
B. C6H5NH2+Br2(aq.)→
Solution
C6H5N2Cl is known as benzenediazonium-chloride. It will undergo a substitution reaction while reacting with hypophosphorous acid .
Complete step by step answer: C6H5N2Cl is known as benzenediazonium chloride. It is a saet of a diazonium cation and chloride. It exists as a colourless solid.
As we know the diazonium group is a very good leaving group and being positively charged, it takes away the two electrons of the bond that it has with the phenyl group. So, the incoming group that replaces N2+ has to be a nucleophile.
-Hence, from H3PO2 the nucleophilic group that can be formed will be H3PO2−. The presence of H2O in the acid can also act as a nucleophile through its oxygen.
The products formed by the reaction of benzenediazonium chloride are C6H6,N2,HCl and H3PO3.
The reaction equation can be written as :-
C6H5N2Cl+H3PO2+H2O→C6H6+N2+H3PO3+HCl
-when C6H5N2Cl that is benzenediazonium chloride reacts with Br2 then the product will be 2,4,6−tribromoaniline the reaction equation involved is :-
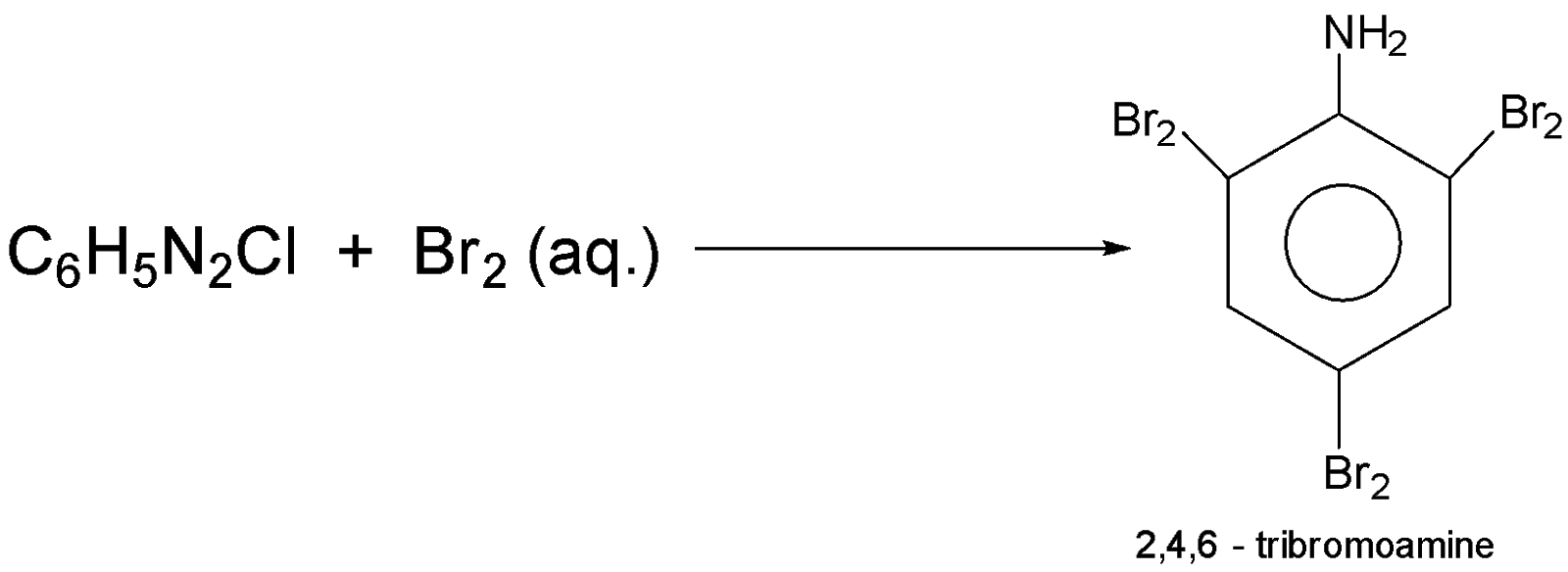
Additional Information: Benzenediazonium chloride is the parent member of the aryl diazonium compound that has use in organic chemistry; this salt is highly unstable.
Note: The diazo group (N2)can be replaced with many other groups, usually anions, that give a variety of substituted phenyl derivatives.
Moreover, this compound seems to be explosive.
