Question
Question: Complete the following reaction- \[C{{H}_{3}}Cl(excess)+N{{H}_{3}}\to\]...
Complete the following reaction-
CH3Cl(excess)+NH3→
Solution
The above reaction is a nucleophilic substitution reaction called Ammonolysis. Since CH3Cl is present in excess, the product would be a mixture of primary, secondary, tertiary amines and also a quaternary ammonium salt.
Complete step by step solution:
We know that the C-X (carbon – halogen) bond in alkyl or benzyl halides can be cleaved by a nucleophile. Thus, an alkyl or benzyl halide on reaction with ammonia undergoes nucleophilic substitution reaction wherein the halogen atom is replaced by an amino (−NH2) group. This process of breaking of the C–X bond by a molecule of ammonia is known as ammonolysis. And this reaction is carried out in a sealed tube at a temperature of 373 K.
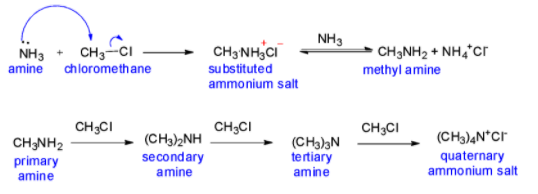
In the next step of the reaction an ammonia molecule may remove one of the hydrogens from the ammonium salt. An ammonium ion is formed, along with a primary amine, methylamine in this case. This reaction is reversible. The product will contain a mixture of methylammonium ions, ammonia, methylamine and ammonium ions.
The primary amine, that is methyl amine (CH3NH2) in this case, thus obtained behaves as a nucleophile and can further react with alkyl halide which is chloromethane (CH3Cl) to form secondary and tertiary amines like dimethylamine and trimethylamine respectively, and finally quaternary ammonium salt as chloromethane is present in excess.
The free amine can be obtained from the ammonium salt by treatment with a strong base as well. As we saw, ammonolysis has the disadvantage of giving a mixture of primary, secondary, tertiary amines and also a quaternary ammonium salt. However, primary amine can be obtained as a major product by taking large amounts of ammonia.
Additional information:
We can predict the reactivity as the order of reactivity of alkyl halides with amines is given as RI>RBr>RCl
Note: It’s important to note which entity is present in excess. The major product would have been methylamine if the ammonia was present in excess instead of chloromethane.
