Question
Question: Complete the following reaction: \({C_6}{H_5}N{H_2} + {(C{H_3}CO)_2} \to \)...
Complete the following reaction:
C6H5NH2+(CH3CO)2→
Solution
Here, we are given two reactants and we need to find out the product which will be formed when these two reactants, that is, aniline and acetic anhydride, will react together. One reactant is aromatic and the other one is the anhydride form of the acetic acid.
Complete answer: Before finding out the product formed, let’s learn something about these two reactants.
One is aniline and the other one is the anhydride of the acetic acid. Now, aniline is an aromatic organic compound having molecular formula C6H5NH2with the molecular weight as 93gmol−1. A molecule of aniline consists of two parts, that is, one is phenyl (C6H5−) which is bonded with an amino group (−NH2). Aniline is a benzene derivative and is electron rich.
The other reactant is acetic anhydride which is also known as glacial acetic acid which simply means water-free acetic acid, that is, there is no water molecule present in it. Acetic anhydride is widely used in chemistry as a chemical reagent. It is a colourless compound. It’s chemical formula is (CH3CO)2 having molecular weight as 102gmol−1.
Now, when these two reactants, which we have discussed above briefly, react together they form acetanilide as a product. Where the acetic acid part is removed in the product and the N-acylation takes place and then we obtain acetanilide as the product and acetic acid as a by-product.
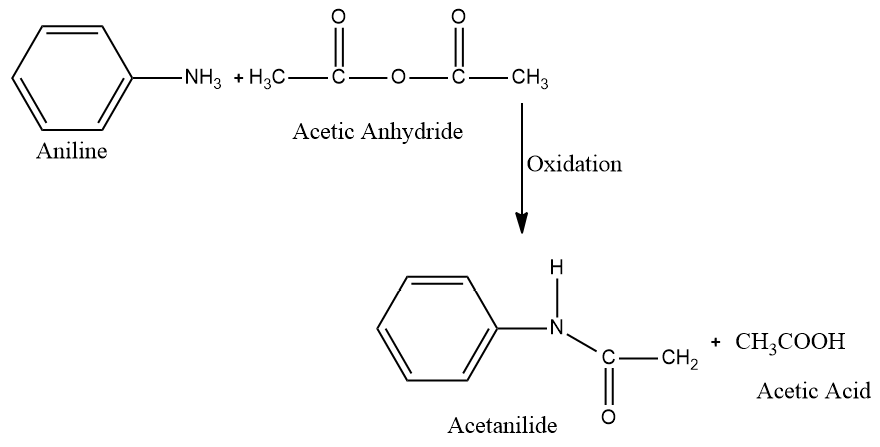
Now, acetanilide is an organic compound having molecular formula C6H5NHCOCH3 and molecular weight as 135g⋅mol−1. It is a white coloured organic compound which is present in solid phase and is used in several organic syntheses.
Note:
Acetanilide is an aniline derivative which has antipyretic properties and also has analgesic properties, that is, acts as a painkiller or pain reliever but it is not used anymore due to its highly toxic effects such as cyanosis which means purple or blue coloured skin leading to liver or kidney damages and so on.
