Question
Question: Complete the following equation \[Xe{{F}_{6}}+{{H}_{2}}O\to \\_\\_\\_\\_\\_+2HF\]...
Complete the following equation
Xe{{F}_{6}}+{{H}_{2}}O\to \\_\\_\\_\\_\\_+2HF
Solution
Hint: Reaction of compounds with water is known as hydrolysis. Hydrolysis products are usually oxides or hydroxides.
Complete step by step solution:
Xenon hexafluoride is a compound of the noble gas Xenon with formula XeF6. It is one of the three binary fluorides of xenon. Xenon hexafluoride is the strongest fluorinating agent and is a colourless solid. The solid readily sublimes into intensely yellow vapours.
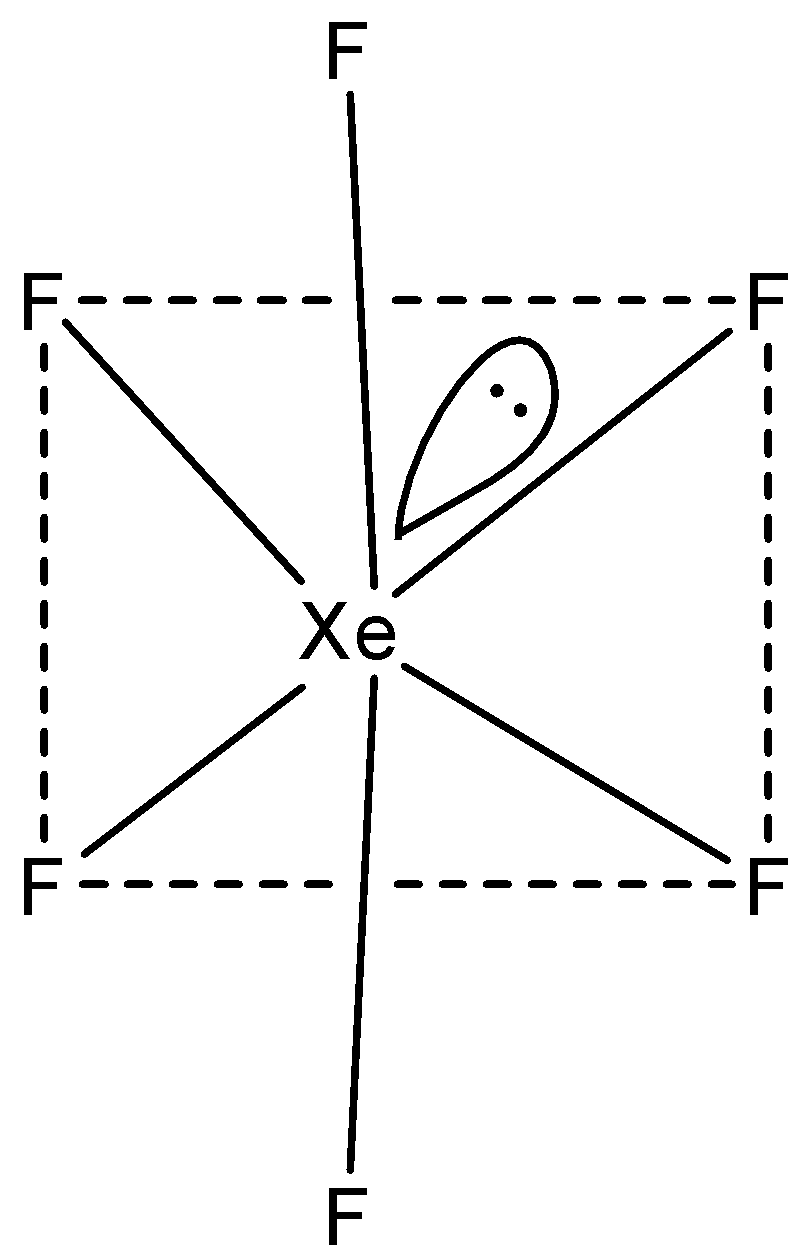
Structure of Xenon hexafluoride is distorted octahedral and has a hybridisation sp3d3.
In the question, hydrolysis of XeF6 is carried out. On hydrolysis we get XeOF4. Further hydrolysis can be carried out to give XeO3. The reaction is as follows,
XeF6+H2O→XeOF4+2HF
On further hydrolysis,
XeOF4+H2O→XeO2F2+2HF
XeO2F2+H2O→XeO3+2HF
The total hydrolysis reaction can be shown as
3XeF6+6H2O→2XeO3+Xe+12HF
Hence, the complete reaction is XeF6+H2O→XeOF4+2HF.
Additional Information:
- Xenon hexafluoride can act as lewis acids too by binding with one or two anions.
-It is prepared by heating xenon difluoride at 300 degree Celsius under 60 atm pressure of fluorine. NiF2 is the catalyst used. These are exergonic (positive flow of energy from system to surroundings) and are stable at normal temperatures.
-Xenon hexafluoride has distorted octahedral structure because of the lone pair of electrons of xenon. All the six fluorides occupy positions just like in normal octahedral structure and the lone pair occupies a position as shown in the figure above.
Note: Note that in the question only 2 moles of HF is produced, so the reaction is the first hydrolysed product. If it was a complete hydrolysis reaction, then the number moles of HF will be 12.
